Summary
Abstract
Liposomal amphotericin B (AmBisome®) is a lipid-associated formulation of the broad-spectrum polyene antifungal agent amphotericin B. It is active against clinically relevant yeasts and moulds, including Candida spp., Aspergillus spp. and filamentous moulds such as Zygomycetes, and is approved for the treatment of invasive fungal infections in many countries worldwide. It was developed to improve the tolerability profile of amphotericin B deoxycholate, which was for many decades considered the gold standard of antifungal treatment, despite being associated with infusion-related events and nephrotoxicity.
In well controlled trials, liposomal amphotericin B had similar efficacy to amphotericin B deoxycholate and amphotericin B lipid complex as empirical therapy in adult and paediatric patients with febrile neutropenia. In addition, caspofungin was noninferior to liposomal amphotericin B as empirical therapy in adult patients with febrile neutropenia. For the treatment of confirmed invasive fungal infections, liposomal amphotericin B was more effective than amphotericin B deoxycholate treatment in patients with disseminated histoplasmosis and AIDS, and was noninferior to amphotericin B deoxycholate in patients with acute cryptococcal meningitis and AIDS. In adults, micafungin was shown to be non-inferior to liposomal amphotericin B for the treatment of candidaemia and invasive candidiasis. Data from animal studies suggested that higher dosages of liposomal amphotericin B might improve efficacy; however, in the AmBiLoad trial in patients with invasive mould infection, there was no statistical difference in efficacy between the standard dosage of liposomal amphotericin B 3 mg/kg/day and a higher 10 mg/kg/day dosage, although the standard dosage was better tolerated.
Despite being associated with fewer infusion-related adverse events and less nephrotoxicity than amphotericin B deoxycholate and amphotericin B lipid complex, liposomal amphotericin B use is still limited to some extent by these adverse events. Both echinocandins were better tolerated than liposomal amphotericin B. The cost of liposomal amphotericin B therapy may also restrict its use, but further pharmacoeconomic studies are required to fully define its cost effectiveness compared with other antifungal agents. Based on comparative data from well controlled trials, extensive clinical experience and its broad spectrum of activity, liposomal amphotericin B remains a first-line option for empirical therapy in patients with febrile neutropenia and in those with disseminated histoplasmosis, and is an option for the treatment of AIDS-associated cryptococcal meningitis, and for invasive Candida spp. or Aspergillus spp. infections.
Pharmacological Properties
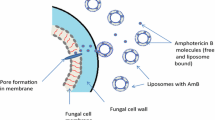
Amphotericin B, a macrocyclic, polyene antifungal agent, is thought to act by binding to ergosterol, the principal sterol in fungal cell membranes and Leishmania cells. This results in a change in membrane permeability, causing metabolic disturbance, leakage of small molecules and, as a consequence, cell death. In vitro and in vivo studies have shown that liposomal amphotericin B remains closely associated with the liposomes in the circulation, thereby reducing the potential for nephrotoxicity and infusion-related toxicity associated with conventional amphotericin B. Amphotericin B shows very good in vitro activity against a broad spectrum of clinically relevant fungal isolates, including most strains of Candida spp. and Aspergillus spp., and other filamentous fungi such as Zygomycetes. Liposomal amphotericin B has proven effective in various animal models of fungal infections, including those for candidiasis, aspergillosis, fusariosis and zygomycosis. Liposomal amphotericin B also shows immuno-modulatory effects, although the mechanisms involved are not fully understood, and differ from those of amphotericin B deoxycholate and amphotericin B colloidal dispersion.
In adult patients with febrile neutropenia, intravenous liposomal amphotericin B has nonlinear pharmacokinetics, with higher than dose-proportional increases in exposure being consistent with reticuloendothelial saturation and redistribution of amphotericin B in the plasma compartment. Liposomal amphotericin B is rapidly and extensively distributed after single and multiple doses, with steady-state concentrations of amphotericin B attained within 4 days and no clinically relevant accumulation of the drug following multiple doses of 1–7.5 mg/kg/day. In autopsy tissue, the highest concentrations of the drug were found in the liver and spleen, followed by the kidney, lung, myocardium and brain tissue. Elimination of liposomal amphotericin B, like that of amphotericin B deoxycholate, is poorly understood; its route of metabolism is not known and its excretion has not been studied. The terminal elimination half-life is about 7 hours. No dosage adjustment is required based on age or renal impairment.
Clinical Efficacy
In several randomized, double-blind trials (n = 73–1095) in adult and/or paediatric patients, liposomal amphotericin B was effective as empirical therapy or as treatment for confirmed invasive fungal infections, including invasive candidiasis, candidaemia, invasive mould infection (mainly aspergillosis), histoplasmosis and cryptococcal meningitis. All agents were administered as an intravenous infusion; the typical dosage for liposomal amphotericin B was 3 mg/kg/day. Treatment was generally given for 1–2 weeks.
Participants in trials evaluating empirical therapy had neutropenia and a persistent fever despite antibacterial treatment and had received chemotherapy or undergone haematopoietic stem cell transplantation. As empirical therapy in adult and paediatric patients, liposomal amphotericin B appeared to be as effective as amphotericin B deoxycholate (approximately 50% of patients in each group achieved treatment success) or amphotericin B lipid complex (approximately 40% of liposomal amphotericin B recipients experienced treatment success). Of note, in the first trial, results of the statistical test to determine equivalence between treatments were not reported. In the second trial, efficacy was assessed as an ‘other’ endpoint. In another trial, caspofungin was shown to be noninferior to liposomal amphotericin B, with approximately one-third of patients in each group experiencing treatment success.
Liposomal amphotericin B was significantly more effective than amphotericin B deoxycholate for the treatment of moderate to severe disseminated histoplasmosis in patients with AIDS, with 88% and 64% of patients, respectively, having a successful response. Liposomal amphotericin B was noninferior to amphotericin B deoxycholate for the treatment of cryptococcal meningitis in terms of mycological success. Micafungin therapy was shown to be noninferior to liposomal amphotericin B for the treatment of adult patients with candidaemia or invasive candidiasis. In a substudy in paediatric patients, which was not powered to determine noninferiority, liposomal amphotericin B was as effective as micafungin for the treatment of candidaemia or invasive candidiasis. In this patient population, within each trial, 90% of adult patients and approximately three-quarters of paediatric patients in both treatment groups experienced a successful response. In patients with invasive mould infection (mainly aspergillosis), there was no difference in efficacy between a higher dosage of liposomal amphotericin B (10mg/kg/day) and the standard dosage (3 mg/kg/day), with 46% and 50% of patients experiencing a favourable overall response.
Tolerability
In well designed clinical trials, liposomal amphotericin B was generally at least as well tolerated as other lipid-associated formulations of amphotericin B and better tolerated than amphotericin B deoxycholate in adult and paediatric patients. Compared with other amphotericin B formulations, liposomal amphotericin B treatment was associated with a lower incidence of infusion-related adverse events and nephrotoxicity. A higher than recommended dosage of liposomal amphotericin B (10 mg/kg/day) was associated with an increased incidence of nephrotoxicity compared with the standard dosage (3 mg/kg/day), although the incidence of infusion-related reactions did not differ between treatment groups.
In general, liposomal amphotericin B treatment was not as well tolerated as echinocandin therapy in well designed clinical trials. As empirical therapy or for the treatment of confirmed invasive fungal infections in adult patients, liposomal amphotericin B recipients experienced more infusion-related events and nephrotoxicity than caspofungin or micafungin recipients. There was no difference in the incidence of these adverse events between the liposomal amphotericin B and micafungin groups in a study in paediatric patients.






Similar content being viewed by others
References
Cornely OA. Aspergillus to zygomycetes: causes, risk factors, prevention, and treatment of invasive fungal infections. Infection 2008 Aug; 36(4): 296–313
Marchetti O, Cordonnier C, Calandra T. Empirical antifungal therapy in neutropaenic cancer patients with persistent fever. Eur J Cancer Suppl 2007; 5(2): 32–42
Olson JA, Adler-Moore JP, Jensen GM, et al. Comparison of the physicochemical, antifungal, and toxic properties of two liposomal amphotericin B products. Antimicrob Agents Chemother 2008 Jan; 52(1): 259–68
Kshirsagar NA, Pandya SK, Kirodian GB, et al. Liposomal drug delivery system from laboratory to clinic. J Postgrad Med 2005; 51 Suppl. 1: S5–15
AmBisome® (liposomal amphotericin B): Irish summary of product characteristics. Gilead Sciences International Limited, 2007 Apr
Coukell AJ, Brogden RN. Liposomal amphotericin B: therapeutic use in the management of fungal infections and visceral leishmaniasis. Drugs 1998 Apr; 55(4): 585–612
Adler-Moore J, Proffitt RT. AmBisome: liposomal formulation, structure, mechanism of action and pre-clinical experience. J Antimicrob Chemother 2002 Feb; 49 Suppl. 1: 21–30
Adler-Moore JP, Proffitt RT. Amphotericin B lipid preparations: what are the differences? Clin Microbiol Infect 2008; 14 Suppl. 4: 25–36
Takemoto K, Yamamoto Y, Ueda Y. Evaluation of antifungal pharmacodynamic characteristics of AmBisome against Candida albicans. Microbiol Immunol 2006; 50(8): 579–86
Torrado JJ, Espada R, Ballesteros MP, et al. Amphotericin B formulations and drug targeting. J Pharm Sci 2008; 97(7): 2405–25
Sabra R, Zeinoun N, Sharaf LH, et al. Role of humoral mediators in, and influence of a liposomal formulation on, acute amphotericin B nephrotoxicity. Pharmacol Toxicol 2001 Apr; 88(4): 168–75
Barchiesi F, Arzeni D, Compagnucci P, et al. In vitro activity of five antifungal agents against clinical isolates of Saccharomyces cerevisiae. Med Mycol 1998 Dec; 36: 437–40
Tzatzarakis MN, Tsatsakis AM, Charvalos E, et al. Comparison of in vitro activities of amphotericin, clotrimazole, econazole, miconazole, and nystatin against Fusarium oxysporum. J Environ Sci Health B 2001 May; 36(3): 331–40
Lewis RE, Wiederhold NP, Klepser ME. In vitro pharmacodynamics of amphotericin B, itraconazole, and voriconazole against Aspergillus, Fusarium, and Scedosporium spp. Antimicrob Agents Chemother 2005 Mar; 49(3): 945–51
Kantarcioğlu AS, Yucel A. In-vitro activities of terbinafine, itraconazole and amphotericin B against Aspergillus and Cladosporium species. J Chemother 2002 Dec; 14(6): 562–7
Lacroix C, de Chauvin MF. In vitro activity of amphotericin B, itraconazole, voriconazole, posaconazole, caspofungin and terbinafine against Scytalidium dimidiatum and Scytalidium hyalinum clinical isolates. J Antimicrob Chemother 2008 Apr; 61(4): 835–7
Torres-Narbona M, Guinea J, Martínez-Alarcón J, et al. In vitro activities of amphotericin B, caspofungin, itraconazole, posaconazole, and voriconazole against 45 clinical isolates of zygomycetes: comparison of CLSI M38-A, Sensititre YeastOne, and the Etest. Antimicrob Agents Chemother 2007 Mar; 51(3): 1126–9
Sun QN, Fothergill AW, McCarthy DI, et al. In vitro activities of posaconazole, itraconazole, voriconazole, amphotericin B, and fluconazole against 37 clinical isolates of zygomycetes. Antimicrob Agents Chemother 2002 May; 46(5): 1581–2
Gómez-López A, Cuenca-Estrella M, Monzón A, et al. In vitro susceptibility of clinical isolates of Zygomycota to amphotericin B, flucytosine, itraconazole and voriconazole. J Antimicrob Chemother 2001 Dec; 48(6): 919–21
Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts; third informational supplement M27-S 3. Wayne (PA): Clinical and Laboratory Standards Institute, 2008; 28 (15)
Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard-third edition M27-A 3. Wayne (PA): Clinical and Laboratory Standards Institute, 2008; 28 (14)
Carrillo-Muñoz AJ, Quindós G, Ruesga M, et al. Antifungal activity of posaconazole compared with fluconazole and amphotericin B against yeasts from oropharyngeal candidiasis and other infections. J Antimicrob Chemother 2005 Mar; 55(3): 317–9
Pemán J, Cantón E, Gobernado M. Epidemiology and antifungal susceptibility of Candida species isolated from blood: results of a 2-year multicentre study in Spain. Eur J Clin Microbiol Infect Dis 2005 Jan; 24(1): 23–30
Chiu YS, Chang SC, Hsueh PR, et al. Survey of amphotericin B susceptibility of Candida clinical isolates determined by Etest. J Microbiol Immunol Infect 2006 Aug; 39(4): 335–41
Diekema DJ, Messer SA, Hollis RJ, et al. Activities of caspofungin, itraconazole, posaconazole, ravuconazole, voriconazole, and amphotericin B against 448 recent clinical isolates of filamentous fungi. J Clin Microbiol 2003 Aug; 41(8): 3623–6
Espinel-Ingroff A, Johnson E, Hockey H, et al. Activities of voriconazole, itraconazole and amphotericin B in vitro against 590 moulds from 323 patients in the voriconazole phase III clinical studies. J Antimicrob Chemother 2008 Mar; 61(3): 616–20
Vitale RG, Meis JF, Mouton JW, et al. Evaluation of the post-antifungal effect (PAFE) of amphotericin B and nystatin against 30 zygomycetes using two different media. J Antimicrob Chemother 2003 Jul; 52(1): 65–70
Di Bonaventura G, Spedicato I, Picciani C, et al. In vitro pharmacodynamic characteristics of amphotericin B, caspofungin, fluconazole, and voriconazole against bloodstream isolates of infrequent Candida species from patients with hematologic malignancies. Antimicrob Agents Chemother 2004 Nov; 48(11): 4453–6
Philip A, Odabasi Z, Rodriguez J, et al. In vitro synergy testing of anidulafungin with itraconazole, voriconazole, and amphotericin B against Aspergillus spp. and Fusarium spp. Antimicrob Agents Chemother 2005 Aug; 49(8): 3572–4
Arikan S, Lozano-Chiu M, Paetznick V, et al. In vitro synergy of caspofungin and amphotericin B against Aspergillus and Fusarium spp. Antimicrob Agents Chemother 2002 Jan; 46(1): 245–7
Kuhn DM, George T, Chandra J, et al. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother 2002 Jun; 46(6): 1773–80
Zarif L, Graybill JR, Perlin D, et al. Antifungal activity of amphotericin B cochleates against Candida albicans infection in a mouse model. Antimicrob Agents Chemother 2000 Jun; 44(6): 1463–9
van Etten EW, Snijders SV, van Vianen W, et al. Superior efficacy of liposomal amphotericin B with prolonged circulation in blood in the treatment of severe candidiasis in leukopenic mice. Antimicrob Agents Chemother 1998 Sep; 42(9): 2431–3
Clemons KV, Espiritu M, Parmar R, et al. Comparative efficacies of conventional amphotericin B, liposomal amphotericin B (AmBisome), caspofungin, micafungin, and voriconazole alone and in combination against experimental murine central nervous system aspergillosis. Antimicrob Agents Chemother 2005 Dec; 49(12): 4867–75
Clemons KV, Stevens DA. Comparative efficacies of four amphotericin B formulations-Fungizone, Amphotec (Amphocil), AmBisome, and Abelcet-against systemic murine aspergillosis. Antimicrob Agents Chemother 2004 Mar; 48(3): 1047–50
Takemoto K, Yamamoto Y, Ueda Y, et al. Comparative study on the efficacy of AmBisome and Fungizone in a mouse model of pulmonary aspergillosis. J Antimicrob Chemother 2006 Apr; 57(4): 724–31
Olson JA, Adler-Moore JP, Schwartz J, et al. Comparative efficacies, toxicities, and tissue concentrations of amphotericin B lipid formulations in a murine pulmonary aspergillosis model. Antimicrob Agents Chemother 2006 Jun; 50(6): 2122–31
Lewis RE, Liao G, Hou J, et al. Comparative analysis of amphotericin B lipid complex and liposomal amphotericin B kinetics of lung accumulation and fungal clearance in a murine model of acute invasive pulmonary aspergillosis. Antimicrob Agents Chemother 2007 Apr; 51(4): 1253–8
Van Etten EW, Stearne-Cullen LE, ten Kate M, et al. Efficacy of liposomal amphotericin B with prolonged circulation in blood in treatment of severe pulmonary aspergillosis in leukopenic rats. Antimicrob Agents Chemother 2000 Mar; 44(3): 540–5
Gavalda` J, Martin T, López P, et al. Efficacy of high loading doses of liposomal amphotericin B in the treatment of experimental invasive pulmonary aspergillosis. Clin Microbiol Infect 2005 Dec; 11(12): 999–1004
Martín MT, Gavaldà J, López P, et al. Efficacy of high doses of liposomal amphotericin B in the treatment of experimental aspergillosis. J Antimicrob Chemother 2003 Dec; 52(6): 1032–4
Takemoto K, Yamamoto Y, Ueda Y, et al. Comparative studies on the efficacy of AmBisome and Fungizone in a mouse model of disseminated aspergillosis. J Antimicrob Chemother 2004 Feb; 53(2): 311–7
Ortoneda M, Capilla J, Pastor FJ, et al. Efficacy of liposomal amphotericin B in treatment of systemic murine fusariosis. Antimicrob Agents Chemother 2002 Jul; 46(7): 2273–5
Ibrahim AS, Gebremariam T, Husseiny MI, et al. Comparison of lipid amphotericin B preparations in treating murine zygomycosis. Antimicrob Agents Chemother 2008 Apr; 52(4): 1573–6
Andes D, Safdar N, Marchillo K, et al. Pharmacokinetic-pharmacodynamic comparison of amphotericin B (AMB) and two lipid-associated AMB preparations, liposomal AMB and AMB lipid complex, in murine candidiasis models. Antimicrob Agents Chemother 2006 Feb; 50(2): 674–84
Groll AH, Giri N, Petraitis V, et al. Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida albicans infection of the central nervous system. J Infect Dis 2000 Jul; 182(1): 274–82
Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 2007; 20(1): 133–63
Barker KS, Crisp S, Wiederhold N, et al. Genome-wide expression profiling reveals genes associated with amphotericin B and fluconazole reistance in experimentally induced antifungal resistant isolates of Candida albicans. J Antimicrob Chemother 2004; 54: 376–85
Blum G, Perkhofer S, Haas H, et al. Potential basis for amphotericin B resistance in Aspergillus terreus. Antimicrob Agents Chemother 2008 Apr; 52(4): 1553–5
Ben-Ami R, Lewis RE, Kontoyiannis DP. Immunopharmacology of modern antifungals. Clin Infect Dis 2008; 47: 226–35
Simitsopoulou M, Roilides E, Dotis J, et al. Differential expression of cytokines and chemokines in human monocytes induced by lipid formulations of amphotericin B. Antimicrob Agents Chemother 2005 Apr; 49(4): 1397–403
Bellocchio S, Gaziano R, Bozza S, et al. Liposomal amphotericin B activates antifungal resistance with reduced toxicity by diverting Toll-like receptor signalling from TLR-2 to TLR-4. J Antimicrob Chemother 2005 Feb; 55(2): 214–22
Pachl J, Svoboda P, Jacobs F, et al. A randomized, blinded, multicenter trial of lipid-associated amphotericin B alone versus in combination with an antibody-based inhibitor of heat shock protein 90 in patients with invasive candidiasis. Clin Infect Dis 2006; 42: 1404–6
Dotis J, Simitsopoulou M, Dalakiouridou M, et al. Amphotericin B formulations variably enhance antifungal activity of human neutrophils and monocytes against Fusarium solani: comparison with Aspergillus fumigatus. J Antimicrob Chemother 2008 Apr; 61(4): 810–7
Roilides E, Lyman CA, Filioti J, et al. Amphotericin B formulations exert additive antifungal activity in combination with pulmonary alveolar macrophages and polymorphonuclear leukocytes against Aspergillus fumigatus. Antimicrob Agents Chemother 2002 Jun; 46(6): 1974–6
Dotis J, Simitsopoulou M, Dalakiouridou M, et al. Effects of lipid formulations of amphotericin B on activity of human monocytes against Aspergillus fumigatus. Antimicrob Agents Chemother 2006 Mar; 50(3): 868–73
Walsh TJ, Yeldandi V, McEvoy M, et al. Safety, tolerance, and pharmacokinetics of a small unilamellar liposomal formulation of amphotericin B (AmBisome) in neutropenic patients. Antimicrob Agents Chemother 1998 Sep; 42(9): 2391–8
Vogelsinger H, Weiler S, Djanani A, et al. Amphotericin B tissue distribution in autopsy material after treatment with liposomal amphotericin B and amphotericin B colloidal dispersion. J Antimicrob Chemother 2006 Jun; 57(6): 1153–60
Groll AH, Mickiene D, Piscitelli SC, et al. Distribution of lipid formulations of amphotericin B into bone marrow and fat tissue in rabbits. Antimicrob Agents Chemother 2000 Feb; 44(2): 408–10
Adler-Moore J, Proffitt RT. Effect of tissue penetration on AmBisome efficacy. Curr Opin Investig Drugs 2003 Feb; 4(2): 179–85
Bellmann R. Clinical pharmacokinetics of systemically administered antimycotics. Curr Clin Pharmacol 2007 Jan; 2(1): 37–58
Bekersky I, Fielding RM, Dressler DE, et al. Plasma protein binding of amphotericin B and pharmacokinetics of bound versus unbound amphotericin B after administration of intravenous liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate. Antimicrob Agents Chemother 2002 Mar; 46(3): 834–40
Walsh TJ, Pappas P, Winston DJ, et al. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N Engl J Med 2002 Jan 24; 346(4): 225–34
Cornely OA, Maertens J, Bresnik M, et al. Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial). Clin Infect Dis 2007 May 15; 44(10): 1289–97
Walsh TJ, Finberg RW, Arndt C, et al. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study Group. N Engl J Med 1999 Mar 11; 340(10): 764–71
Wingard JR, White MH, Anaissie E, et al. A randomized, double-blind comparative trial evaluating the safety of liposomal amphotericin B versus amphotericin B lipid complex in the empirical treatment of febrile neutropenia. L Amph/ABLC Collaborative Study Group. Clin Infect Dis 2000 Nov; 31(5): 1155–63
Walsh TJ, Teppler H, Donowitz GR, et al. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N Engl J Med 2004 Sep 30; 351(14): 1391–402
Johnson PC, Wheat LJ, Cloud GA, et al. Safety and efficacy of liposomal amphotericin B compared with conventional amphotericin B for induction therapy of histoplasmosis in patients with AIDS. Ann Intern Med 2002 Jul 16; 137(2): 105–9
Kuse ER, Chetchotisakd P, da Cunha CA, et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 2007 May 5; 369(9572): 1519–27
Queiroz-Telles F, Berezin E, Leverger G, et al. Micafungin versus liposomal amphotericin B for pediatric patients with invasive candidiasis: substudy of a randomized double-blind trial. Pediatr Infect Dis J 2008 Sep; 27(9): 820–6
Hamill RJ, Sobel J, El-Sadr W, et al. Randomized doubleblind trial of AmBisome (liposomal amphotericin B) and amphotericin B in acute cryptococcal meningitis in AIDS patients [abstract no. 1161]. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1999 Sep 26–29; San Francisco (CA)
US Food and Drugs Administration Center for Drug Evaluation and Research. AmBisome (Amphotericin B) injection: medical review NDA 50-740/SE1-002 [online]. Available from URL: http://www.fda.gov/cder/foi/nda/2000/50-740s2_AmBisome_medr.pdf [Accessed 2009 Jan 19]
Goldman RD, Koren G. Amphotericin B nephrotoxicity in children. J Pediatr Hematol Oncol 2004 Jul; 26(7): 421–6
Dupont B. Overview of the lipid formulations of amphotericin B. J Antimicrob Chemother 2002 Feb; 49 Suppl. 1: 31–6
Gilead Sciences. Optimising treatment with amphotericin B: importance of correct dosing for patients receiving amphotericin B products. Gilead Sciences, 2008 Jul. (Data on file)
Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009 Mar 1; 48(5): 503–35
Denning DW, Kibbler CC, Barnes RA. British Society for Medical Mycology proposed standards of care for patients with invasive fungal infections. Lancet Infect Dis 2003 Apr; 3(4): 230–40
Saag MS, Graybill RJ, Larsen RA, et al. Practice guidelines for the management of cryptococcal disease. Infectious Diseases Society of America. Clin Infect Dis 2000 Apr; 30(4): 710–8
Herbrecht R, Flückiger U, Gachot B, et al. Treatment of invasive Candida and invasive Aspergillus infections in adult haematological patients. Eur J Cancer Suppl 2007; 5(2): 49–59
Wingard JR. Empirical antifungal therapy in treating febrile neutropenic patients. Clin Infect Dis 2004 Jul 15; 39 Suppl. 1: S38–43
British Committee for Standards in Haematology. Guidelines on the management of invasive fungal infection during therapy for haematological malignancy [online]. Available from URL: http://www.bcshguidelines.com/pdf/IFI_therapy.pdf [Accessed 2008 Sep 9]
Rüping MJ, Vehreschild JJ, Cornely OA. Patients at high risk of invasive fungal infections: when and how to treat. Drugs 2008; 68(14): 1941–62
Rüping MJ, Vehreschild JJ, Cornely OA. Antifungal treatment strategies in high risk patients. Mycoses 2008 Sep; 51 Suppl. 2: 46–51
Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2008 Feb 1; 46(3): 327–60
Wheat J, Sarosi G, McKinsey D, et al. Practice guidelines for the management of patients with histoplasmosis. Infectious Diseases Society of America. Clin Infect Dis 2000 Apr; 30(4): 688–95
Jørgensen KJ, Gøtzsche PC, Johansen HK. Voriconazole versus amphotericin B in cancer patients with neutropenia. Cochrane Database Syst Rev 2006 Jan 25; (1): CD004707
Powers JH, Dixon CA, Goldberger MJ. Voriconazole versus liposomal amphotericin B in patients with neutropenia and persistent fever [letter]. N Engl J Med 2002 Jan 24; 346(4): 289–90
Johnson JR. Voriconazole versus liposomal amphotericin B for empirical antifungal therapy [letter]. N Engl J Med 2002 May 30; 346(22): 1745–7; author reply 1745-7
Ullmann AJ, Heussel CP, Cornely OA. Voriconazole versus liposomal amphotericin B for empirical antifungal therapy [letter]. N Engl J Med 2002 May 30; 346(22): 1745–7; author reply 1745-7
Walsh TJ, Lee J, Dismukes WE. Decisions about voriconazole versus liposomal amphotericin B [letter]. N Engl J Med 2002 May 9; 346 (19): 1499; author reply 1499
Powers JH, Dixon CA, Goldberger M. Decisions about voriconazole versus liposomal amphotericin B [letter]. N Engl J Med 2002 May 9; 346(19): 1499
Bennett JE, Powers J, Walsh T, et al. Forum report: issues in clinical trials of empirical antifungal therapy in treating febrile neutropenic patients. Clin Infect Dis 2003 Apr 15; 36 Suppl. 3: S117–22
de Pauw BE, Sable CA, Walsh TJ, et al. Impact of alternate definitions of fever resolution on the composite endpoint in clinical trials of empirical antifungal therapy for neutropenic patients with persistent fever: analysis of results from the Caspofungin Empirical Therapy Study. Transpl Infect Dis 2006 Mar; 8(1): 31–7
Walsh TJ, Pappas PG, Winston DJ. Voriconazole versus liposomal amphotericin B for empirical antifungal therapy [letter]. N Engl J Med 2002 May 30; 346(22): 1746–7
European Medicines Agency. Assessment report for mycamine (micafungin) [online]. Available from URL: http://www.emea.europa.eu/humandocs/PDFs/EPAR/mycamine/H-734-en6.pdf [Accessed 2009 Feb 9]
Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 2002 Aug 8; 347(6): 408–15
Blot F, Edé C, Nitenberg GM. Voriconazole versus amphotericin B for invasive aspergillosis [letter]. N Engl J Med 2002 Dec 19; 347(25): 2080–1; author reply 2080-1
Karthaus M. Voriconazole versus amphotericin B for invasive aspergillosis [letter]. N Engl J Med 2002 Dec 19; 347(25): 2080–1; author reply 2080-1
Herbrecht R, Patterson TF, Bennett JE. Voriconazole versus amphotericin B for invasive aspergillosis [letter]. N Engl J Med 2002 Dec 19; 347(25): 2080–1
Agarwal R, Singh N. Amphotericin B is still the drug of choice for invasive aspergillosis [letter]. Am J Respir Crit Care Med 2006 Jul 1; 174(1): 102; author reply 102-3
Denning DW. Comparison of 2 studies of treatment of invasive aspergillosis [letter]. Clin Infect Dis 2007 Oct 15; 45(8): 1106–8; author reply 1108-10
White MH, Bowden RA, Sandler ES, et al. Randomized, double-blind clinical trial of amphotericin B colloidal dispersion vs. amphotericin B in the empirical treatment of fever and neutropenia. Clin Infect Dis 1998 Aug; 27(2): 296–302
Bowden R, Chandrasekar P, White MH, et al. A double-blind, randomized, controlled trial of amphotericin B colloidal dispersion versus amphotericin B for treatment of invasive aspergillosis in immunocompromised patients. Clin Infect Dis 2002 Aug 15; 35(4): 359–66
Rogers TR. Treatment of zygomycosis: current and new options. J Antimicrob Chemother 2008 Jan; 61 Suppl. 1: i35–9
Cross SA, Scott LJ. Micafungin: a review of its use in adults for the treatment of invasive and oesophageal candidiasis, and as prophylaxis against Candida infections. Drugs 2008; 68(15): 2225–55
Scott LJ, Simpson D. Voriconazole: a review of its use in the management of invasive fungal infections. Drugs 2007; 67(2): 269–98
McCormack PL, Perry CM. Caspofungin: a review of its use in the treatment of fungal infections. Drugs 2005; 65(14): 2049–68
Astellas Pharma US Inc. AmBisome® (amphotericin B) liposome for injection: US prescribing information [online]. Available from URL: http://www.fda.gov/cder/foi/label/2008/050740s016lbl.pdf [Accessed 2008 Dec 9]
E. R. Squibb & Sons Limited. Fungizone intravenous: UK summary of product characteristics [online]. Available from URL: http://emc.medicines.org.uk/medicine/559/SPC/Fungizone+Intravenous/ [Accessed 2009 Feb 24]
Cephalon Limited. Abelcet®: UK summary of product characteristics [online]. Available from URL: http://emc.medicines.org.uk/medicine/2133/SPC/Abelcet/ [Accessed 2009 Feb 24]
Beacon Pharmaceuticals. Amphocil™: UK summary of product characteristics [online]. Available from URL: http://emc.medicines.org.uk/medicine/18416/SPC/Amphocil+50mg/ [Accessed 2009 Feb 24]
Almirante B, Rodríguez D. Antifungal agents in neonates: issues and recommendations. Paediatr Drugs 2007; 9(5): 311–21
Leibovitz E. Neonatal candidosis: clinical picture, management controversies and consensus, and new therapeutic options. J Antimicrob Chemother 2002 Feb; 49 Suppl. 1: 69–73
Frattarelli DA, Reed MD, Giacoia GP, et al. Antifungals in systemic neonatal candidiasis. Drugs 2004; 64(9): 949–68
Kaskel P, Tuschy S, Wagner A, et al. Economic evaluation of caspofungin vs liposomal amphotericin B for empirical therapy of suspected systemic fungal infection in the German hospital setting. Ann Hematol 2008 Apr; 87(4): 311–9
Bruynesteyn K, Gant V, McKenzie C, et al. A cost-effectiveness analysis of caspofungin vs. liposomal amphotericin B for treatment of suspected fungal infections in the UK. Eur J Haematol 2007 Jun; 78(6): 532–9
Cornely OA, Sidhu M, Odeyemi I, et al. Economic analysis of micafungin versus liposomal amphotericin B for treatment of candidaemia and invasive candidiasis in Germany. Curr Med Res Opin 2008 May 9; 24(6): 1743–53
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: J. Adler-Moore, Department of Biological Sciences, California State Polytechnic University, Pomona, California, USA; R.A. Barnes, Department of Medical Microbiology, Cardiff University School of Medicine, Cardiff, Wales; O.A. Cornely, Department I of Internal Medicine and Clinical Trials Center, University of Cologne, Cologne, Germany; D.W. Denning, School of Translational Medicine, Wythenshawe Hospital and the University of Manchester, Manchester, England; R. Herbrecht, Hematology and Oncology Department, University Hospital of Strasbourg, Strasbourg, France; C. Lass-Flörl, Department of Hygiene, Microbiology and Social Medicine, Innsbruck Medical University, Innsbruck, Austria.
Data Selection
Sources: Medical literature published in any language since 1980 on ‘amphotericin B liposomal’, identified using MEDLINE and EMBASE, supplemented by AdisBase (a proprietary database of Wolters Kluwer Health | Adis). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: MEDLINE, EMBASE and AdisBase search terms were ‘amphotericin B liposomal’ or ‘liposomal amphotericin B’. Searches were last updated 23 February 2009.
Selection: Studies in patients with febrile neutropenia or invasive fungal infections who received liposomal amphotericin B. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Liposomal amphotericin B, febrile neutropenia, invasive fungal infections, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability.
Rights and permissions
About this article
Cite this article
Moen, M.D., Lyseng-Williamson, K.A. & Scott, L.J. Liposomal Amphotericin B. Drugs 69, 361–392 (2009). https://doi.org/10.2165/00003495-200969030-00010
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200969030-00010