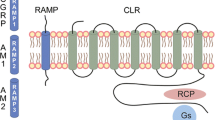
Abstract
Calcitonin gene-related peptide (CGRP) is a potent neuromodulator that is expressed in the trigeminovascular system and is released into the cranial circulation in various primary headaches. CGRP is released in migraine, cluster headache and paroxysmal hemicrania. The blockade of its release is associated with the successful treatment of acute migraine and cluster headache. CGRP receptor blockade has recently been shown to be an effective acute anti-migraine strategy and is non-vasoconstricting in terms of the mechanism of action. The prospect of a non-vasoconstricting therapy for acute migraine offers a real opportunity to patients, and perhaps more importantly, provides a therapeutic rationale to reinforce migraine as a neurological disorder.

Similar content being viewed by others
References
Lipton RB, Stewart WF, Diamond S, et al. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache 2001; 41: 646–57
Menken M, Munsat TL, Toole JF. The global burden of disease study: implications for neurology. Arch Neurol 2000; 57: 418–20
Goadsby PJ, Lipton RB, Ferrari MD. Migraine: current understanding and treatment. N Engl J Med 2002; 346: 257–70
Lance JW, Goadsby PJ. Mechanism and management of headache. 7th ed. New York: Elsevier, 2005
Goadsby PJ. The pharmacology of headache. Prog Neurobiol 2000; 62: 509–25
Doenicke A, Siegel E, Hadoke M, et al. Initial clinical study of AH25086B (5-HT1-like agonist) in the acute treatment of migraine. Cephalalgia 1987; 7: 437–8
Doenicke A, Brand J, Perrin VL. Possible benefit of GR43175, a novel 5-HT1-like receptor agonist, for the acute treatment of severe migraine. Lancet 1988; I: 1309–11
Ferrari MD, The Subcutaneous Sumatriptan International Study Group. Treatment of migraine attacks with sumatriptan. N Engl J Med 1991; 325: 316–21
Dodick D, Lipton RB, Martin V, et al. Consensus statement: cardiovascular safety profile of triptans (5-HT1B/1D agonists) in the acute treatment of migraine. Headache 2004; 44: 414–25
Ferrari MD, Roon KI, Lipton RB, et al. Oral triptans (serotonin, 5-HT1B/1D agonists) in acute migraine treatment: a meta-analysis of 53 trials. Lancet 2001; 358: 1668–75
Ferrari MD, Goadsby PJ, Roon KI, et al. Triptans (serotonin, 5-HT1B/1D agonists) in migraine: detailed results and methods of a meta-analysis of 53 trials. Cephalalgia 2002; 22: 633–58
Humphrey PPA, Goadsby PJ. Controversies in headache: the mode of action of sumatriptan is vascular? A debate. Cephalalgia 1994; 14: 401–10
Amara SG, Jonas V, Rosenfeld MG, et al. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature 1982; 298: 240–4
Amara SG, Arriza JL, Leff SE, et al. Expression in brain of a messenger RNA encoding a novel neuropeptide homologous to calcitonin gene-related peptide. Science 1985; 229: 1094–7
Chan-Palay V, Edvinsson L. Innervation of cerebral blood vessels by norepinephrine indolamine substance P and neurotensin fibres and the leptomeningeal indolamine axons: their roles in vasomotor activity and local alterations of brain blood composition. In: Owman C, editor. Neurogenic control of the brain circulation. Sydney: Pergamon Press, 1977: 39–53
Wolff HG. Headache and other head pain. 1st ed. New York: Oxford University Press, 1948
Edvinsson L, McCulloch J, Uddman R. Substance P: immunohistochemical localization and effect upon cat pial arteries in vitro and in situ. J Physiol 1981; 318: 251–8
Uddman R, Edvinsson L, Owman C, et al. Perivascular substance P: occurrence and distribution in mammalian pial vessels. J Cereb Blood Flow Metab 1981; 1: 227–32
Uddman R, Edvinsson L, Ekman R, et al. Innervation of the feline cerebral vasculature by nerve fibers containing calcitonin gene-related peptide: trigeminal origin and co-existence with substance P. Neurosci Lett 1985; 62: 131–6
Liu-Chen LY, Han DH, Moskowitz MA. Pia arachnoid contains substance P originating from trigeminal neurons. Neuroscience 1983; 9: 803–8
Liu-Chen LY, Mayberg MR, Moskowitz MA. Immunohistochemical evidence for a substance P-containing trigeminovascular pathway to pial arteries in cats. Brain Res 1983; 268: 162–6
Moskowitz MA, Brody M, Liu-Chen LY. In vitro release of immunoreactive substance P from putative afferent nerve endings in bovine pia arachnoid. Neuroscience 1983; 9: 809–14
Edvinsson L, Rosendahl-Helgesen S, Uddman R. Substance P: localization, concentration and release in cerebral arteries, choroid plexus and dura mater. Cell Tissue Res 1983; 234: 1–7
Liu-Chen L–Y, Gillespie SA, Norregaard TV, et al. Co-localization of retrogradely transported wheat germ agglutinin and the putative neurotransmitter substance P within trigeminal ganglion cells projecting to cat middle cerebral. J Comp Neurol 1984; 225: 187–92
Uddman R, Hara H, Edvinsson L. Neuronal pathways to the rat middle meningeal artery revealed by retrograde tracing and immunocytochemistry. J Auton Nerv Syst 1989; 26: 69–75
Edvinsson L, Hara H, Uddman R. Retrograde tracing of nerve fibers to the middle cerebral artery with true blue: co-localization with different peptides. J Cereb Blood Flow Metab 1989; 9: 212–8
Moskowitz MA, Reinhard JF, Romero J, et al. Neurotransmitters and the fifth cranial nerve: is there a relation to the headache phase of migraine? Lancet 1979; II: 883–4
Edvinsson L, McCulloch J, Kingman TA, et al. On the functional role of the trigemino-cerebrovascular system in the regulation of cerebral circulation. In: Owman C, Hardebo JE, editors. Neural regulation of the cerebral circulation. Stockholm: Elsevier Science Publishers BV, 1986: 407–18
McCulloch J, Uddman R, Kingman TA, et al. Calcitonin generelated peptide: functional role in cerebrovascular regulation. Proc Natl Acad Sci U S A 1986; 83: 1–5
Lambert GA, Bogduk N, Goadsby PJ, et al. Decreased carotid arterial resistance in cats in response to trigeminal stimulation. J Neurosurg 1984; 61: 307–15
Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptides in the trigeminovascular system of man. Proceedings of the 10th International Congress of Pharmacology. Copenhagen: Elsevier Science Publishers, 1987: O319
Goadsby PJ, Edvinsson L, Ekman R. Extracerebral levels of circulating vasoactive peptides during migraine headache. Cephalalgia 1989; 9 Suppl. 10: 292–3
Arbab MA-R, Wiklund L, Svendgaard NA. Origin and distribution of cerebral vascular innervation from superior cervical, trigeminal and spinal ganglia investigated with retrograde and anterograde WGA-HRP tracing in the rat. Neuroscience 1986; 19: 695–708
Liu Y, Zhang M, Broman J, et al. Central projections of sensory innervation of the rat superficial temporal artery. Brain Res 2003; 966: 126–33
Liu Y, Broman J, Edvinsson L. Central projections of sensory innervation of the rat superior sagittal sinus. Neuroscience 2004; 129: 431–7
Goadsby PJ, Edvinsson L, Ekman R. Release of vasoactive peptides in the extracerebral circulation of man and the cat during activation of the trigeminovascular system. Ann Neurol 1988; 23: 193–6
O’Connor TP, van der Kooy D. Enrichment of a vasoactive neuropeptide (calcitonin gene related peptide) in trigeminal sensory projection to the intracranial arteries. J Neurosci 1988; 8: 2468–76
O’Connor TP, van der Kooy D. Pattern of intracranial and extracranial projections of trigeminal ganglion cells. J Neurosci 1986; 6: 2200–7
Feindel W, Penfield W, McNaughton F. The tentorial nerves and localization of intracranial pain in man. Neurology 1960; 10: 555–63
Ray BS, Wolff HG. Experimental studies on headache: pain sensitive structures of the head and their significance in headache. Arch Surg 1940; 41: 813–56
Penfield W, McNaughton FL. Durai headache and the innervation of the dura mater. Arch Neurol Psychiatry 1940; 44: 43–75
McNaughton FL. The innervation of the intracranial blood vessels and durai sinuses. Proc Assoc Res Nerv Ment Dis 1938; 18: 178–200
McNaughton FL, Feindel WH. Innervation of intracranial structures: a reappraisal. In: Rose FC, editor. Physiological aspects of clinical neurology. Oxford: Blackwell Scientific Publications, 1977: 279–93
Rowbottom GI. Observations on the effects of V denervation. Brain 1939; 62: 364–80
Oka M. Experimental study on the vasodilator innervation of the face. Med J Osaka Univ 1950; 2: 109–16
Sweet WM, Wepsic JG. Controlled thermocoagulation of V ganglion and rootlets for differential destruction of pain fibres. Part I: V neuralgia. J Neurosurg 1974; 40: 143–56
Onofrio BM. Radiofrequency percutaneous Gasserian ganglion lesions: results in 140 patients with trigeminal pain. J Neurosurg 1975; 42: 132–43
Drummond PD, Gonski A, Lance JW. Facial flushing after thermocoagulation of the gasserian ganglion. J Neurol Neurosurg Psychiatry 1983; 46: 611–6
Gonzalez G, Onofrio BM, Kerr FWL. Vasodilator system for the face. J Neurosurg 1975; 42: 696–703
Goadsby PJ, Lambert GA, Lance JW. Stimulation of the trigeminal ganglion increases flow in the extracerebral but not the cerebral circulation of the monkey. Brain Res 1986; 381: 63–7
Tran-Dinh YR, Thurel C, Cunin G, et al. Cerebral vasodilation after the thermocoagulation of the trigeminal ganglion in humans. Neurosurgery 1992; 31: 658–62
Goadsby PJ, Lambert GA, Lance JW. The peripheral pathway for extracranial vasodilatation in the cat. J Auton Nerv Syst 1984; 10: 145–55
Goadsby PJ, Macdonald GJ. Extracranial vasodilatation mediated by VIP (vasoactive intestinal polypeptide). Brain Res 1985; 329: 285–8
Goadsby PJ, Duckworth JW. Effect of stimulation of trigeminal ganglion on regional cerebral blood flow in cats. Am J Physiol 1987; 253: R270–4
Goadsby PJ, Knight YE, Hoskin KL, et al. Stimulation of an intracranial trigeminally-innervated structure selectively increases cerebral blood flow. Brain Res 1997; 751: 247–52
Goadsby PJ, Seylaz J, Mraovitch S. Hypercapnic but not neurogenic cortical vasodilatation is blocked by spreading depression in rat. In: lesen J, editor. Migraine and other headaches: the vascular mechanisms. Frontiers in Headache Research, Vol. 1. New York: Raven Press, 1991: 181–6
Macfarlane R, Tasdemiroglu E, Moskowitz MA, et al. Chronic trigeminal ganglionectomy or topical capsaicin application to pial vessels attenuates postocculsive cortical hyperemia but does not influence postischemia hypoperfusion. J Cereb Blood Flow Metab 1991; 11: 261–71
Sakas DE, Moskowitz MA, Wei EP, et al. Trigeminovascular fibers increase blood flow in cortical grey matter by axon-dependent mechanisms during severe hypertension or seizures. Proc Natl Acad Sci U S A 1989; 86: 1401–5
Markowitz S, Saito K, Moskowitz MA. Neurogenically mediated leakage of plasma proteins occurs from blood vessels in dura mater but not brain. J Neurosci 1987; 7: 4129–36
Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev 2004; 84: 903–34
Tarn C, Brain SD. The assessment of vasoactive properties of CGRP and adrenomedullin in the microvasculature: a study using in vivo and in vitro assays in the mouse. J Mol Neurosci 2004; 22: 117–24
Grant AD, Tam CW, Lazar Z, et al. The calcitonin gene-related peptide (CGRP) receptor antagonist BIBN4096BS blocks CGRP and adrenomedullin vasoactive responses in the microvasculature. Br J Pharmacol 2004; 142: 1091–8
Kandere-Grzybowska K, Gheorghe D, Priller J, et al. Stress-induced dura vascular permeability does not develop in mast cell-deficient and neurokinin-1 receptor knockout mice. Brain Res 2003; 980: 213–20
Grant AD, Pinter E, Salmon AM, et al. An examination of neurogenic mechanisms involved in mustard oil-induced inflammation in the mouse. Eur J Pharmacol 2005; 507: 273–80
Maltos KL, Menezes GB, Caliari MV, et al. Vascular and cellular responses to pro-inflammatory stimuli in rat dental pulp. Arch Oral Biol 2004; 49: 443–50
Polley JS, Gaskin PJ, Perren MJ, et al. The activity of GR205171, a potent non-peptide tachykinin NK1 receptor antagonist, in the trigeminovascular system. Regul Pept 1997; 68: 23–9
Goldstein DJ, Offen WW, Klein EG, et al. Lanepitant, an NK-1 antagonist, in migraine prevention. Cephalalgia 2001; 21: 102–6
Goldstein DJ, Wang O, Saper JR, et al. Ineffectiveness of neurokinin-1 antagonist in acute migraine: a crossover study. Cephalalgia 1997; 17: 785–90
Diener H-C, The RPR100893 Study Group. RPR100893, a substance-P antagonist, is not effective in the treatment of migraine attacks. Cephalalgia 2003; 23: 183–5
Connor HE, Bertin L, Gillies S, et al. The GR205171 Clinical Study Group. Clinical evaluation of a novel, potent, CNS penetrating NK1 receptor antagonist in the acute treatment of migraine [abstract]. Cephalalgia 1998; 18: 392
Norman B, Panebianco D, Block GA. A placebo-controlled, inclinic study to explore the preliminary safety and efficacy of intravenous L-758,298 (a prodrug of the NK1 receptor antagonist L-754,030) in the acute treatment of migraine [abstract]. Cephalalgia 1998; 18: 407
May A, Goadsby PJ. Substance P receptor antagonists in the therapy of migraine. Expert Opin Investig Drugs 2001; 10: 1–6
May A, Gijsman HJ, Wallnoefer A, et al. Endothelin antagonist bosentan blocks neurogenic inflammation, but is not effective in aborting migraine attacks. Pain 1996; 67: 375–8
Gupta P, Brown D, Butler P, et al. The in vivo pharmacological profile of a 5-HT1 receptor agonist, CP122,288, a selective inhibitor of neurogenic inflammation. Br J Pharmacol 1995; 116: 2385–90
Roon KI, Olesen J, Diener HC, et al. No acute antimigraine efficacy of CP-122,288, a highly potent inhibitor of neurogenic inflammation: results of two randomized double-blind placebo-controlled clinical trials. Ann Neurol 2000; 47: 238–41
Giles H, Honey A, Edvinsson L, et al. Pre-clinical pharmacology of 4991W93, a potent inhibitor of neurogenic plasma protein extravasation [abstract]. Cephalalgia 1999; 19: 402
Earl NL, McDonald SA, Lowy MT, et al. Efficacy and tolerability of the neurogenic inflammation inhibitor, 4991W93, in the acute treatment of migraine [abstract]. Cephalalgia 1999; 19: 357
Data J, Britch K, Westergaard N, et al. A double-blind study of ganaxolone in the acute treatment of migraine headaches with or without an aura in premenopausal females [abstract]. Headache 1998; 38: 380
Williamson DJ, Hargreaves RJ, Hill RG, et al. Intravital microscope studies on the effects of neurokinin agonists and calcitonin gene-related peptide on durai blood vessel diameter in the anaesthetized rat. Cephalalgia 1997; 17: 518–24
Akerman S, Williamson DJ, Hill RG, et al. The effect of adrenergic compounds on neurogenic durai vasodilation. Eur J Pharmacol 2001; 424: 53–8
Goadsby PJ, Hoskin KL. The distribution of trigeminovascular afferents in the nonhuman primate brain Macaca nemestrina: a c-fos immunocytochemical study. J Anat 1997; 190: 367–75
Akerman S, Williamson D, Hill RG, et al. The effect of adrenergic compounds on neurogenic durai vasodilatation. Eur J Pharmacol 2001; 424: 53–8
Akerman S, Williamson DJ, Kaube H, et al. The role of histamine in durai vessel dilation. Brain Res 2002; 956: 96–102
Kowacs F, Williamson DJ, Goadsby PJ. Neurogenic vasodilation of durai blood vessels is not mediated by cholinergic transmission in the anaesthetised rat. Eur J Pharmacol 2004; 493: 133–7
Williamson DJ, Hargreaves RJ, Hill RG, et al. Sumatriptan inhibits neurogenic vasodilation of durai blood vessels in the anaesthetized ratintravital microscope studies. Cephalalgia 1997; 17: 525–31
Williamson DJ, Shepheard SL, Hill RG, et al. The novel anti-migraine agent rizatriptan inhibits neurogenic durai vasodilation and extravasation. Eur J Pharmacol 1997; 328: 61–4
Leone M, Grazzi L, Mantia LL, et al. Flunarizine in migraine: a mini review. Headache 1991; 31: 388–91
Akerman S, Kaube H, Goadsby PJ. The effect of anti-migraine compounds on nitric oxide induced dilation of durai meningeal vessels. Eur J Pharmacol 2002; 452: 223–8
Diener HC, Tfelt-Hansen P, Dahlof C, et al. Topiramate in migraine prophylaxis: results from a placebo-controlled trial with propranolol as an active control. J Neurol 2004; 251: 943–50
Akerman S, Goadsby PJ. Topiramate inhibits trigeminovascular activation: an intravital microscopy study. Br J Pharmacol 2005 Sep; 146(1): 7–14
Thomsen LL, Krause C, Iversen HK, et al. A nitric oxide donor (nitroglycerine) triggers genuine migraine attacks. Eur J Neurol 1994; 1: 73–80
Ekbom K. Nitroglycerin as a provocative agent in cluster headache. Arch Neurol 1968; 19: 487–93
Akerman S, Williamson DJ, Kaube H, et al. Nitric oxide synthase inhibitors can antagonise neurogenic and calcitonin gene-related peptide induced dilation of durai meningeal vessels. Br J Pharmacol 2002; 137: 62–8
Lassen LH, Ashina M, Christiansen I, et al. Nitric oxide synthesis inhibition in migraine. Lancet 1997; 349: 401–2
Matsuda LA, Lolait SJ, Brownstein MJ, et al. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990; 346: 561–4
Hoehe MR, Caenazzo L, Martinez MM, et al. Genetic and physical mapping of the human cannabinoid receptor gene to chromosome 6q14-q 15. New Biol 1991; 3: 880–5
Devane WA, Harms L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992; 258: 1946–9
Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993; 365: 61–5
Akerman S, Kaube H, Goadsby PJ. Anandamide is able to inhibit trigeminal neurons using an in vivo model of trigeminovascular-mediated nociception. J Pharmacol Exp Ther 2004; 309: 56–63
Gunthorpe MJ, Benham CD, Randall A, et al. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol Sci 2002; 23: 183–91
Akerman S, Kaube H, Goadsby PJ. Anandamide shows both cannabinoid and vanilloid properties in an in vivo model of trigeminovascular mediated headpain [abstract]. Cephalalgia 2003; 23: 646
Akerman S, Kaube H, Goadsby PJ. Vanilloid type 1 receptor (VR1) evoked CGRP release plays a minor role in causing durai vessel dilation via the trigeminovascular system. Br J Pharmacol 2003; 140: 718–24
Edvinsson L, Jansen I, Kingman TA, et al. Cerebrovascular responses to capsaicin in vitro and in situ. Br J Pharmacol 1990; 100: 312–8
Hou M, Uddman R, Tajti J, et al. Capsaicin receptor immunoreactivity in the human trigeminal ganglion. Neurosci Lett 2002; 330: 223–6
Moreno MJ, Abounader R, Hebert E, et al. Efficacy of the non-peptide CGRP receptor antagonist BIBN4096BS in blocking CGRP-induced dilations in human and bovine cerebral arteries: potential implications in acute migraine treatment. Neuropharmacology 2002; 42: 568–76
Doods H, Hallermayer G, Wu D, et al. Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br J Pharmacol 2000; 129: 420–3
Petersen KA, Lassen LH, Birk S, et al. The effect of the nonpeptide CGRP-antagonist, BIBN406BS on human-alphaCGRP induced headache and hemodynamics in healthy volunteers [abstract]. Cephalalgia 2003; 23: 725
Storer RJ, Akerman S, Goadsby PJ. Calcitonin gene-related peptide (CGRP) modulates nociceptive trigeminovascular transmission in the cat. Br J Pharmacol 2004; 142: 1171–81
Storer RJ, Goadsby PJ. Microiontophoretic application of serotonin (5HT)1B/1D agonists inhibits trigeminal cell firing in the cat. Brain 1997; 120: 2171–7
Lambert GA, Lowy AJ, Boers P, et al. The spinal cord processing of input from the superior sagittal sinus: pathway and modulation by ergot alkaloids. Brain Res 1992; 597: 321–30
Kaube H, Hoskin KL, Goadsby PJ. Activation of the trigeminovascular system by mechanical distension of the superior sagittal sinus in the cat. Cephalalgia 1992; 12: 133–6
Cumberbatch MJ, Hill RG, Hargreaves RJ. Rizatriptan has central antinociceptive effects against durally evoked responses. Eur J Pharmacol 1997; 328: 37–40
Kaube H, Hoskin KL, Goadsby PJ. Intravenous acetylsalicylic acid inhibits central trigeminal neurons in the dorsal horn of the upper cervical spinal cord in the cat. Headache 1993; 33: 541–50
Hoskin KL, Kaube H, Goadsby PJ. Central activation of the trigeminovascular pathway in the cat is inhibited by dihydroergotamine: a c-Fos and electrophysiology study. Brain 1996; 119: 249–56
Kaube H, Hoskin KL, Goadsby PJ. Inhibition by sumatriptan of central trigeminal neurones only after blood-brain barrier disruption. Br J Pharmacol 1993; 109: 788–92
Goadsby PJ, Hoskin KL. Inhibition of trigeminal neurons by intravenous administration of the serotonin (5HT)1B/D receptor agonist zolmitriptan (311C90): are brain stem sites a therapeutic target in migraine? Pain 1996; 67: 355–9
Goadsby PJ, Knight YE. Inhibition of trigeminal neurons after intravenous administration of naratriptan through an action at the serotonin (5HT1B/1D) receptors. Br J Pharmacol 1997; 122: 918–22
Goadsby PJ, Hoskin KL. Differential effects of low dose CP122,288 and eletriptan on fos expression due to stimulation of the superior sagittal sinus in cat. Pain 1999; 82: 15–22
Boers P, Donaldson C, Zagami AS, et al. 5-HT1A and 5-HT1B/ 1D receptors are involved in the modulation of the trigeminovascular system of the cat: a microiontophoretic study. Neuropharmacology 2000; 39: 1833–47
Donaldson C, Boers PM, Hoskin KL, et al. The role of 5-HT1B and 5-HT1D receptors in the selective inhibitory effect of naratriptan on trigeminovascular neurons. Neuropharmacology 2002; 42: 374–85
Boers PM, Donaldson C, Zagami AS, et al. Naratriptan has a selective inhibitory effect on trigeminovascular neurones at central 5-HT1A and 5-HT(lB/lD) receptors in the cat: implications for migraine therapy. Cephalalgia 2004; 24: 99–109
Storer RJ, Akerman S, Connor HE, et al. 4991W93, a potent blocker of neurogenic plasma protein extravasation, inhibits trigeminal neurons at 5-hydroxytryptamine (5-HT1B/1D) agonist doses. Neuropharmacology 2001; 40: 911–7
Buzzi MG, Moskowitz MA, Shimizu T, et al. Dihydroergotamine and sumatriptan attenuate levels of CGRP in plasma in rat superior sagittal sinus during electrical stimulation of the trigeminal ganglion. Neuropharmacology 1991; 30: 1193–200
Arvieu L, Mauborgne A, Bourgoin S, et al. Sumatriptan inhibits the release of CGRP and substance P from the rat spinal cord. Neuroreport 1996; 7: 1973–6
Goadsby PJ, Edvinsson L. Peripheral and central trigeminovascular activation in cat is blocked by the serotonin (5HT)-1D receptor agonist 311C 90. Headache 1994; 34: 394–9
Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol 1993; 33: 48–56
Zagami AS, Goadsby PJ, Edvinsson L. Stimulation of the superior sagittal sinus in the cat causes release of vasoactive peptides. Neuropeptides 1990; 16: 69–75
Knight YE, Edvinsson L, Goadsby PJ. Blockade of CGRP release after superior sagittal sinus stimulation in cat: a comparison of avitriptan and CP122, 288. Neuropeptides 1999; 33: 41–6
Knight YE, Edvinsson L, Goadsby PJ. 4991W93 inhibits release of calcitonin gene-related peptide in the cat but only at doses with 5HT1B/1D receptor agonist activity. Neuropharmacol 2001; 40: 520–5
Durham PL, Sharma RV, Russo AF. Repression of the calcitonin gene-related peptide promoter by 5-HT1 receptor activation. J Neurosci 1997; 17: 9545–53
Durham PL, Russo AF. Regulation of calcitonin gene-related peptide secretion by a serotonergic antimigraine drug. J Neurosci 1999; 19: 3423–9
Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol 1990; 28: 183–7
Gallai V, Sarchielli P, Floridi A, et al. Vasoactive peptides levels in the plasma of young migraine patients with and without aura assessed both interictally and ictally. Cephalalgia 1995; 15: 384–90
Afridi S, Kaube H, Goadsby PJ. Glyceryl trinitrate triggers premonitory symptoms in migraineurs. Pain 2004; 110: 675–80
Juhasz G, Zsombok T, Modos EA, et al. NO-induced migraine attack: strong increase in plasma calcitonin gene-related peptide (CGRP) concentration and negative correlation with platelet serotonin release. Pain 2003; 106: 461–70
Juhasz G, Zsombok T, Jakab B, et al. Sumatriptan causes parallel decrease in plasma calcitonin gene-related peptide (CGRP) concentration and migraine headache during nitroglycerin induced migraine attack. Cephalalgia 2005; 25: 179–83
Headache Classification Committee of the International Headache Society. The international classification of headache disorders. 2nd ed. Cephalalgia 2004; 24 Suppl. 1: 1–160
Goadsby PJ, Edvinsson L. Human in vivo evidence for trigeminovascular activation in cluster headache. Brain 1994; 117: 427–34
Fanciullacci M, Alessandri M, Figini M, et al. Increase in plasma calcitonin gene-related peptide from extracerebral circulation during nitroglycerin-induced cluster headache attack. Pain 1995; 60: 119–23
Goadsby PJ, Edvinsson L. Neuropeptide changes in a case of chronic paroxysmal hemicrania-evidence for trigemino-parasympathetic activation. Cephalalgia 1996; 16: 448–50
Ashina M, Bendtsen L, Jensen R, et al. Plasma levels of calcitonin gene-related peptide in chronic tension-type headache. Neurology 2000; 55: 1335–40
Lipton RB, Stewart WF, Cady R, et al. Sumatriptan for the range of headaches in migraine sufferers: results of the Spectrum Study. Headache 2000; 40: 783–91
Tfelt-Hansen P, Saxena PR, Dahlof C, et al. Ergotamine in the acute treatment of migraine: a review and European consensus. Brain 2000; 123: 9–18
Goadsby PJ, Edvinsson L. Dihydroergotamine inhibits trigeminovascular activation in vivo [abstract]. Headache 1992; 32: 255
Olesen J, Diener H-C, Husstedt I-W, et al. Calcitonin gene-related peptide (CGRP) receptor antagonist BIBN4096BS is effective in the treatment of migraine attacks. N Engl J Med 2004; 350: 1104–10
Acknowledgements
The work of the author has been supported by the Wellcome Trust. The author has advised or collaborated with the manufacturers of the triptans and Boehringer-Ingelheim.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goadsby, P.J. Calcitonin Gene-Related Peptide Antagonists as Treatments of Migraine and Other Primary Headaches. Drugs 65, 2557–2567 (2005). https://doi.org/10.2165/00003495-200565180-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200565180-00002