Summary
Abstract
Trastuzumab is a humanised monoclonal antibody developed to target the HER2 receptor which is overexpressed by some cancer cells, including 25 to 30% of breast cancers. Binding with high affinity to the extracellular domain of HER2, trastuzumab inhibits the proliferation of tumour cells that overexpress HER2.
A large well designed multicentre study found that the addition of trastuzumab to either an anthracycline plus cyclophosphamide or to paclitaxel, as first-line therapy for metastatic breast cancer overexpressing the HER2 receptor, significantly increased time to disease progression, rate of objective response, duration of response and survival compared with chemotherapy alone.
Single-agent trastuzumab was associated with an objective response in 15% of extensively pretreated patients with metastatic breast cancer overexpressing HER2, and 26% of previously untreated patients. Patients with a HER2 over-expression level of 3+ using immunohistochemical (IHC) assay or a positive HER2 result using fluorescence in situ hybridisation (FISH), benefit more from trastuzumab therapy than those with tumours overexpressing at a level of 2+.
Trastuzumab has demonstrated synergistic action with several chemotherapy agents preclinically but the optimal combination clinically is yet to be determined.
Trastuzumab is generally well tolerated by most patients; the most significant adverse effects being acute fever and/or chills and the potential to cause cardiac dysfunction. Serious adverse events, including anaphylaxis and death, have occurred in 0.25% of patients.
Symptomatic or asymptomatic cardiac dysfunction occurred in 27% of patients receiving an anthracycline and cyclophosphamide combined with trastuzumab. Thus, combination therapy with anthracyclines is not recommended. Symptomatic or asymptomatic cardiac dysfunction occurred in 13% of patients receiving trastuzumab plus paclitaxel and in 4.7% of patients receiving trastuzumab alone.
Conclusion: Intravenous trastuzumab is effective as a single-agent, and in combination with chemotherapy it significantly improves the median time to disease progression and survival time in patients with metastatic breast cancer overexpressing the HER2 receptor compared with chemotherapy alone. Cardiotoxicity is the main concern with therapy; particularly in patients with pre-existing cardiac dysfunction, the elderly and in combination with, or following, anthracyclines. Trastuzumab is indicated for use with paclitaxel as first-line therapy or as a single agent in second- or third-line treatment regimens for patients with metastatic breast cancer overexpressing HER2. Investigation is ongoing to ascertain the optimal combination regimen containing trastuzumab and antineoplastic agents. In addition, current research is focusing on the optimal timing, sequencing and duration of therapy as well as administration in the neoadjuvant and adjuvant setting.
Pharmacodynamic Properties
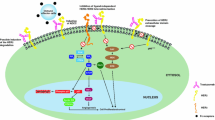
The HER2/neu gene encodes a 185-kd transmembrane glycoprotein receptor (HER2) that has intrinsic tyrosine kinase activity and belongs to the epidermal growth factor receptor family. The HER2 receptor is overexpressed in 25 to 30% of breast cancers and is associated with a poor prognosis. The murine monoclonal antibody (MAb) 4D5, directed against HER2, specifically inhibits the growth of human cancer cells that overexpress the HER2 receptor. MAb 4D5 is humanised by replacing all the mouse-derived components, except the antigen-binding region, with human counterparts. The resulting MAb, trastuzumab, has a high affinity for the extracellular domain (ECD) of HER2 with a similar potency to the murine antibody to inhibit growth of cancer cells (SK-BR-3 cells) overexpressing HER2.
Dose-dependent antitumour activity was observed when trastuzumab was given to nude athymic mice bearing BT-474 human breast cancer cell xenografts overexpressing HER2. Doses ranging from 0.1 to 1 mg/kg intraperitoneally twice a week for 5 weeks resulted in a mean inhibition of tumour growth at 5 weeks of 25 to 80% compared with that in mice treated with a control antibody of nonspecific recombinant MAb immunoglobulin. Trastuzumab caused a similar growth inhibition of tumour volume at 5 weeks to that caused by single-agent doxorubicin or paclitaxel.
In combination studies in mice, treatment with intraperitoneal trastuzumab plus intravenous paclitaxel or doxorubicin caused greater inhibition of tumour growth than any single-agent therapy. Trastuzumab plus paclitaxel inhibited tumour growth more than combination treatment with intravenous doxorubicin.
Trastuzumab, in combination with doxorubicin, cyclophosphamide, methotrexate, etoposide or vinblastine significantly reduced tumour volume when administered to athymic mice bearing HER2-transfected MCF7 human breast cancer xenografts compared with single-agent therapy.
In vitro synergistic cytotoxic activity was demonstrated when trastuzumab was combined with cisplatin, thiotepa and etoposide in SK-BR-3 human breast cancer cells, and additive interactions were observed with paclitaxel, doxorubicin, methotrexate and vinblastine.
Pharmacokinetic Properties
Trastuzumab demonstrated dose-dependent pharmacokinetics with short duration weekly intravenous infusions up to 500mg. At standard doses of 4 mg/kg initially followed by 2 mg/kg/wk, steady-state serum concentrations are produced at approximately 20 weeks. The estimated mean area under the concentration-time curve was 578 mg/L · day and the mean maximum serum concentration and mean minimum serum concentration (Cmin) were approximately 110 and 66 mg/L, respectively.
Detectable levels of baseline circulating ECD HER2 were observed in 64% of 447 patients (mean =11 μg/L) with metastatic breast cancer overexpressing the HER2 receptor. Higher levels of ECD HER2 were associated with a lower Cmin value of trastuzumab. However, most patients with elevated ECD HER2 receptor levels achieved target serum concentration of trastuzumab by week 6.
In all clinical trials of patients with metastatic breast cancer, weekly intravenous infusions of trastuzumab produced a volume of distribution which approximated that of serum volume (2.95L). As only minimal amounts of trastuzumab penetrate into the cerebrospinal fluid, a response to trastuzumab would not be expected in patients with meningeal or cerebral metastases resulting from metastatic breast cancer overexpressing the HER2 receptor.
The mean serum elimination half-life (t½) increased and clearance decreased with increasing doses of intravenous trastuzumab administered once a week to patients with metastatic breast cancer. At standard doses of 4 mg/kg initially then 2 mg/kg/wk, the mean t½ was approximately 28.5 days with a washout period of up to 20 weeks. The mean clearance in clinical trials, following standard doses, was 0.225 L/day.
The disposition of trastuzumab in elderly patients and patients with renal impairment was not altered. No formal drug interaction studies have been performed with trastuzumab.
Clinical Efficacy
A large well designed, multicentre, randomised, single-blind trial compared the efficacy of trastuzumab combined with chemotherapy with that of chemotherapy alone in 469 women with metastatic breast cancer overexpressing the HER2 receptor. None had received previous chemotherapy for metastatic disease. Doxorubicin (or epirubicin) and cyclophosphamide were given to patients who had not previously received adjuvant (postoperative) therapy with an anthracycline, with or without trastuzumab. Patients who had previously received adjuvant anthracycline were treated with paclitaxel with or without trastuzumab.
The addition of intravenous trastuzumab to either regimen was associated with a significantly longer time to disease progression. Patients receiving trastuzumab in combination with either type of chemotherapy showed disease progression at a median time of 7.4 months versus 4.6 months for either type of chemotherapy alone. A significantly higher rate of objective (complete plus partial) response, a longer duration of response, a longer survival and a lower incidence of death during the first year were also observed in trastuzumab-treated groups compared with chemotherapy alone. Combination therapy with trastuzumab lowered the relative risk of death by 20% at a median follow-up of 30 months.
Despite patients aged >60 years appearing to have a less favourable overall clinical outcome than patients aged ≤60 years, there was still a survival benefit in this older group.
In several phase II noncomparative trials (most reported in abstracts), trastuzumab, in combination with a range of chemotherapy agents, has demonstrated clinical efficacy in patients with metastatic breast cancer overexpressing the HER2 receptor.
Data from noncomparative studies suggest that objective response rates to trastuzumab therapy are higher in previously untreated patients than in those who have received extensive prior therapy for metastatic disease.
Single-agent trastuzumab produced a response in 15% of 222 extensively pretreated patients with metastatic breast cancer overexpressing the HER2 receptor (intent-to-treat analysis). The median duration of response was 9.1 months and the median duration of survival was 13 months. Low and high dosages of trastuzumab monotherapy, in a study of previously untreated patients, demonstrated a similar objective response rate in both treatment groups of the intent-to-treat population (overall 26%). A higher response was observed in patients with an HER2 overexpression level of 3+ by immunohistochemical (IHC) assay (35%), and those who were HER2-positive by fluorescence in situ hybridisation (FISH) method of assay (41%).
Data from other clinical trials have also demonstrated that patients with a HER2 overexpression level of 3+ using IHC assay or a positive HER2 result using FISH, benefit more from trastuzumab therapy than those with tumours over-expressing at a level of 2+ (IHC assay). Recent reports suggest that FISH may be a better method for selecting patients for trastuzumab therapy than IHC; particularly those with an overexpression level of 2+. However, strict adherence to test protocols and quality control programmes may improve the consistency of IHC results.
Trastuzumab, alone or in combination with chemotherapy, was associated with an improvement in global quality of life in all three studies assessing patients who participated in the major clinical trials. The level of fatigue also improved in patients receiving trastuzumab, alone or in combination with chemotherapy, as first-line treatment for metastatic breast cancer compared with baseline or with chemotherapy alone.
Tolerability
Trastuzumab, administered intravenously as a single agent or in combination with chemotherapy, is tolerated well by most patients. The most significant adverse events are acute fever and/or chills and a potential to cause symptomatic or asymptomatic cardiac dysfunction.
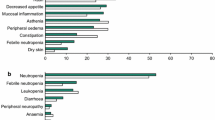
The most common adverse events (incidence >35%) associated with trastuzumab monotherapy are fever, chills, pain, asthenia and nausea. In most cases events are mild to moderate. Fever and/or chills occurred during or shortly after the first infusion of trastuzumab monotherapy in 40% of patients (n = 222), but recurred in less than 3% and usually responded to treatment with paracetamol (acetaminophen) and diphenhydramine. Compared with chemotherapy, trastuzumab was well tolerated and typical chemotherapy-induced events, such as alopecia, mucositis and haematologic toxicity, occurred in no more than 4% of patients.
Adverse events which occurred in >40% of 234 patients receiving intravenous trastuzumab in combination with standard doses of chemotherapy (anthracycline and cyclophosphamide or paclitaxel) include nausea, pain, asthenia, alopecia, fever, diarrhoea, headache, infection, vomiting, leucopenia, dyspnoea and increased cough. Most events were mild or moderate in intensity and led to treatment withdrawal in 25 patients (19 in the group receiving an anthracycline, cyclophosphamide and trastuzumab, and six in the group receiving paclitaxel and trastuzumab).
Infection (mild to moderate in intensity) occurred in 47% of patients who received chemotherapy plus trastuzumab compared with 29% of patients given chemotherapy alone.
Cardiac dysfunction (symptomatic or asymptomatic and generally categorised as >10% reduction in ejection fraction or cardiomyopathy) occurs in some patients receiving trastuzumab as monotherapy or as a component of combination therapy. The incidence was greater in patients receiving trastuzumab in combination with an anthracycline and cyclophosphamide (27%) than in patients receiving an anthracycline and cyclophosphamide alone (8%). 13% of 91 women treated with trastuzumab and paclitaxel, and 1% of 95 patients receiving paclitaxel alone developed symptomatic or asymptomatic cardiac dysfunction. Cardiac function improved in 75% of patients after the initiation of standard medical care.
Trastuzumab monotherapy was associated with an incidence of 4.7% of cardiac dysfunction in women with metastatic breast cancer overexpressing the HER2 receptor. Patients heavily pretreated with anthracyclines, those with preexisting systolic dysfunction and those over 65 years of age are more likely to experience symptoms of systolic dysfunction.
Serious adverse events, including death, have occurred in 0.25% of patients treated with trastuzumab. Serious events fall into three categories: infusion reaction; hypersensitivity reaction, including fatal anaphylaxis; and pulmonary events, including adult respiratory distress syndrome. In most cases symptoms occurred during or immediately after the infusion or during the first 24 hours after administration. An increased risk of a fatal infusion reaction may exist in patients experiencing dyspnoea at rest due to complications of advanced malignancy and comorbidities.
Dosage and Administration
Trastuzumab is indicated for patients with metastatic breast cancer overexpressing the HER2 receptor. In combination with paclitaxel, intravenous trastuzumab is indicated for first-line treatment. In patients who have previously received one or more regimens of chemotherapy for metastatic disease, trastuzumab can be used as a single agent.
The recommended initial loading dose of trastuzumab is 4 mg/kg administered via intravenous infusion over 90 minutes. A weekly dose of 2 mg/kg follows, given over 30 minutes if the initial infusion was well tolerated.
Trastuzumab administration can cause symptomatic or asymptomatic cardiac dysfunction and extreme caution should be taken in treating patients with symptomatic heart failure, a history of hypertension, or documented coronary artery disease. Before therapy with trastuzumab, patients should have a thorough cardiac assessment and cardiac function should be monitored frequently throughout treatment. In clinical trials, the incidence of cardiac dysfunction was highest in patients receiving trastuzumab in combination with an anthracycline and cyclophosphamide and, for this reason, trastuzumab is currently not approved in combination with anthracyclines except in a well controlled setting with cardiac monitoring.
As trastuzumab may remain in the circulation for up to approximately 18 weeks after treatment is stopped, it is recommended that anthracycline therapy should be avoided for up to 22 weeks after therapy is discontinued.
Trastuzumab may be given in an outpatient setting but patients should be observed for infusion-related symptoms, most commonly fever and chills. Rarely, hypersensitivity reactions including anaphylaxis and pulmonary events have also been reported. If patients experience dyspnoea or clinically significant hypotension the infusion should be interrupted and the patient monitored until symptoms resolve. In the case of anaphylaxis, angioedema or acute respiratory distress syndrome, the infusion should be terminated. Trastuzumab is contraindicated in patients with severe dyspnoea at rest due to complications of advanced malignancy or those requiring supplementary oxygen therapy.
No well controlled studies exist of the use of trastuzumab in pregnant women, and therapy should only be initiated if clearly indicated.













Similar content being viewed by others
References
American Cancer Society. What are the key statistics for breast cancer [online]. Available fromURL: http://www.cancer.org [Accessed Sep 2001]
Peto R, Boreham J, Clarke M, et al. UK and USA breast cancer deaths down 25% in year 2000 at ages 20–69 years [letter]. Lancet 2000 May 20; 355: 1822
Hortobagyi GN. Treatment of breast cancer. N Engl J Med 1998 Oct 1; 339: 974–84
Park JW, Tripathy D, Campbell MJ, et al. Biological therapy of breast cancer. Biodrugs 2000 Oct; 14: 221–46
Schechter AL, Stern DF, Vaidyanathan L, et al. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature 1984 Dec 6; 312(5994): 513–6
Albanell J, Baselga J. Trastuzumab, a humanized anti-HER2 monoclonal antibody, for the treatment of breast cancer. Drugs Today 1999; 35(12): 931–46
Press MF, Bernstein L, Thomas PA, et al. HER-2/neu gene amplification characterized by fluorescence in situ hybridization: poor prognosis in node-negative breast carcinomas. J Clin Oncol 1997 Aug; 15(8): 2894–904
Hudziac RM, Schlessinger J, Ullrich A. Increased expression of the putative growth factor receptor p185HER2causes transformation and tumorigenesis of NIH 3T3 cells. Proc Natl Acad Sci U S A 1987; 84(20): 7159–63
Perry CM, Wiseman LR. Trastuzumab. Biodrugs 1999 Aug; 12: 129–35
Carter P, Presta L, Gorman CM, et al. Humanization of an anti-p185HER2antibody for human cancer therapy. Proc Natl Acad Sci U S A 1992 May; 89 (Immunology): 4285–9
Eisenhauer EA. From the molecule to the clinic–inhibiting HER2 to treat breast cancer [editorial]. N Engl J Med 2001 Mar 15; 344(11): 841–2
Treish I, Schwartz R, Lindley C. Pharmacology and therapeutic use of trastuzumab in breast cancer. Am J Health System Pharm 2000 Nov 15; 57(22): 2063–79
Molina MA, Codony-Servat J, Albaneil J, et al. Trastuzumab (Herceptin), a humanized anti-HER2 receptor monoclonal antibody, inhibits basal and activated HER2 ectodomain cleavage in breast cancer cells. Cancer Res 2001 Jun 15; 61(12): 4744–9
Baselga J, Norton L, Albaneil J, et al. Recombinant humanized anti-HER2 antibody (Herceptin®) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu over-expressing human breast cancer xenografts. Cancer Res 1998 Jul 1; 58: 2825–31
Pegram M, Hsu S, Lewis G, et al. Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene 1999 Apr 1; 18: 2241–51
Lopez AM, Pegram MD, Landaw EM, et al. Models to assess combination therapy interactions: optimal timing of anti-HER-2 antibody and doxorubicin in breast cancer [abstract no. 4061]. Proceedings of the American Association for Cancer Research 1997 Mar; 38: San Diego (CA), 605
Park JW, Colbern G, Nuijens A, et al. Increased levels of circulating HER2 ECD in response to anti-HER2 antibody therapy [abstract no. 267]. Breast Cancer Res Treat 1997 Oct; 46 (Special issue): 67
Vogel CL, Cobleigh MA, Tripathy D, et al. First-line herceptin® monotherapy in metastatic breast cancer. Oncology 2001; 61 Suppl. 2: 37–42
Simon R, Nocito A, Hübscher T, et al. Patterns of HER-2/neu amplification and overexpression in primary and metastatic breast cancer. J Natl Cancer Inst 2001 Aug 1; 93(15): 1141–6
Colbern GT, Hiller AJ, Musterer RS, et al. Antitumor activity of Herceptin® in combination with STEALTH® liposomal cisplatin or nonliposomal cisplatin in a HER2 positive human breast cancer model. J Inorg Biochem 1999 Oct; 77: 117–20
Kopreski MS, Lipton A, Harvery H, et al. Growth inhibition of breast cancer cell lines by combinations of anti-P185HER2 monoclonal antibody and cytokines. Anticancer Res 1996; 16: 433–6
Witters LM, Kumar R, Chinchilli VM, et al. Enhanced anti-proliferative activity of the combination of tamoxifen plus HER-2-neu antibody. Breast Cancer Res Treat 1997; 42: 1–5
Kunisue H, Kurebayashi J, Otsuki T, et al. Anti-HER2 antibody enhances the growth inhibitory effect of anti-oestrogen on breast cancer cells expressing both oestrogen receptors and HER 2. Br J Cancer 2000 Jan; 82(1): 46–51
Liu L, Lloyd M, Pegram MD, et al. CP461 induces apoptosis and growth inhibition of breast cancer cells independent of HER2/neu receptor expression [abstract]. Breast Cancer Res Treat 2000 Nov; 64: 94
Normanno N, Campiglio M, De Luca A, et al. Cooperative inhibitory effect of ZD1839 (‘Iressa’) in combination with trastuzumab on human breast cancer cell growth [abstract no. 4156]. Proc Am Assoc Cancer Res 2001 Mar; 42: 774
Tokuda Y, Watanabe T, Omuro Y, et al. Dose escalation and pharmacokinetic study of a humanized anti-HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. Br J Cancer 1999 Dec; 81: 1419–25
Baselga J, Tripathy D, Mendelsohn J, et al. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol 1996 Mar; 14(3): 737–44
Genentech Inc. Herceptin® (trastuzumab): full prescribing information. Sep, 2000
Roche Products Ltd. Herceptin®: Summary of Product Characteristics. 2001. (Data on file)
Gelmon K, Arnold A, Verma S, et al. Pharmacokinetics (PK) and safety of trastuzumab (Herceptin®) when administered every three weeks to women with metastatic breast cancer [abstract no. 271]. Proc Am Soc Clin Oncol 2001 May 12; 20 (Pt 1): 69a
Pegram MD, Lipton A, Hayes DF, et al. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-pl85HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol 1998 Aug; 16(8): 2659–71
Pestalozzi BC, Brignoli S. Herceptin®(trastuzumab) in cerebrospinal fluid. Eur J Cancer 2000 Sep; 36 Suppl. 5: 54
EMEA. EMEA public statement on trastuzumab (Herceptin®): new pharmacokinetic data. 13 Jun, 2001
Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER 2. N Engl J Med 2001 Mar 15; 344: 783–92
Bums III HA. Docetaxel (Taxotere) plus trastuzumab (Herceptin) in breast cancer. Semin Oncol 2001 Feb; 28(1) Suppl. 3: 38–44
Uber KA, Nicholson BP, Thor AD, et al. A phase II trial of weekly docetaxel (D) and Herceptin (H) as first- or secondline treatment in HER2 over-expressing metastatic breast cancer [abstract no. 1949]. Proc Am Soc Clin Oncol 2001 May 12; 20(2): 50
Burris III HA, Hainsworth JD, Miranda FT, et al. Phase II trial of Herceptin induction followed by combination therapy with paclitaxel and carboplatin: a Minnie Pearl Research Network trial [abstract]. Breast Cancer Res Treat 2000; 64: 31
Miller KD, Sisk J, Ansari R, et al. Gemcitabine, paclitaxel, and trastuzumab in metastatic breast cancer. Oncology 2001 Feb; 15(2) Suppl. 3: 38–40
Nabholtz JM, Pienkowski T, Nothfelt D, et al. Results of two open label multicentre phase II pilot studies with Herceptin in combination with docetaxel and platinum salts (Cis or Carboplatin) (TCH) as therapy for advanced breast cancer (ABC) in women with tumors over-expressing the HER2-neu protooncogene [abstract no. 695]. Eur J Cancer 2001 Oct; 37 Suppl. 6: 190
Tsavdaridis D, Timotheadou E, Christodoulou C, et al. Weekly administration of paclitaxel, as first line chemotherapy, and Herceptin (transtuzumab) in advanced breast cancer [abstract]. Ann Oncol 2000; 11 Suppl. 4: 32
Meden H, Beneke A, Hesse T, et al. Weekly intravenous recombinant humanized anti-P185HER2 monoclonal antibody (herceptin) plus docetaxel in patients with metastatic breast cancer: a pilot study. Anticancer Res 2001; 21: 1301–6
Bangemann N, Kuhle A, Ebert A, et al. Capecitabine combined with trastuzumab in the therapy of intensively pretreated HER2-overexpressing metastatic breast cancer (MBC) [abstract]. Ann Oncol 2000; 11 Suppl. 4: 143
Pienkowski T, Fumoleau P, Eiermann J, et al. Taxotere, cisplatin and herceptin (TCH) in first-line HER2 positive metastatic breast cancer (MBC) patients, a phase II pilot study by the breast cancer international research group (BCIRG 101) [abstract no. 2030]. Proc Am Soc Clin Oncol 2001 May 12; 20 (Pt 2): San Francisco, CA
Jahanzeb M, Mortimer J, Yunus D, et al. A phase II multicenter trial of weekly herceptin with navelbine in chemonaive patients with her2 positive metastatic breast cancer [abstract no. 710]. Eur J Cancer 2001 Oct 21; 37 Suppl. 6: 194
Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 1999 Sep; 17: 2639–48
Scidman AD, Former MN, Esteva FJ, et al. Weekly trastuzumab and paclitaxel therapy for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype and gene amplification. J Clin Oncol 2001 May; 19(10): 2587–95
Burstein HJ, Kuter I, Campos SM, et al. Clinical activity of trastuzumab and vinorelbine in women with HER2-overexpressing metastatic breast cancer. J Clin Oncol 2001 May 15; 19(10): 2722–30
Fyfe GA, Mass R, Murphy M, et al. Survival benefit of trastuzumab (Herceptin) and chemotherapy in older (age>60) patients [abstract no. 189]. Proceedings of the 37th Annual Meeting of the American Society of Clinical Oncology; 2001 May 12–15; San Francisco (CA), 48
Krasna L, Janku F, Petruzelka L, et al. Herceptin (H) and Taxol (T) in the treatment of women with HER-2/neu overexpressing metastatic breast cancer (MBC): prospective study [abstract no. 2006]. Proc Am Soc Clin Oncol 2001; May 12; 20(2): 64
Krzemieniecki K, Pawlicki M. Herceptin and Taxol combination for metastatic breast cancer in women with HER-2/neu overexpression [abstract]. 11th Int Congress on Anti-Cancer Treatment; 2001 Feb 6; Paris, 276
Moreau L, Mouret-Reynier MA, Penault-Llorca F, et al. Addition of herceptin to paclitaxel for Her2 over-expressing advanced breast cancer. Best results in skin metastasis [abstract no. P114]. 11th Int Congress on Anti-Cancer Treatment; 2001 Feb 6; Paris, 220–1
Scholz U, Lück HJ, Schippert C, et al. Trastuzumab (Herceptin) combined with weekly paclitaxel in the treatment of metastatic breast cancer: a phase II study [abstract]. Breast Cancer Res Treat 2000 Nov; 64: 122
Verma S, Leyland-Jones B, Ayoub J-P, et al. Efficacy and safety of three weekly herceptin with paclitaxel in women with Her2-positive metastatic breast cancer: preliminary results of a phase II trial [abstract no. 538]. Eur J Cancer 2001 Oct 21; 37 Suppl. 6: S146
Yeung K, Gupta R, Haidak D, et al. Weekly (W) Herceptinr (H, Traztuximab) and one hour Taxol (T, paclitaxel) infusion (WHT) regimen for human epidermal growth factor receptor-2 (HER2) overexpressed (+) metastatic breast cancer (MBC) [abstract]. Proc 36th Am Soc Clin Oncol; 2000 May 20; New Orleans; 19: 142a
Esteva FJ, Valero V, Booser D, et al. Phase II study of weekly docetaxel (Taxotere; txt) and trastuzumab (Herceptin; H) for patients with HER-2 overexpressing (HER2+) metastatic breast cancer (MBC) [abstract no. 706]. Eur J Cancer 2001 Oct 21; 37 Suppl. 6: S193
Kuzur ME, Albain KS, Huntington SF, et al. A phase II trial of docetaxel and Herceptin in metastatic breast cancer patients overexpressing HER-2 [abstract no. 512]. Proc Am Soc Clin Oncol 2000 May 20; 19: 131a
Bangemann N, Kuhle A, Willrodt RG, et al. Treatment of HER2 overexpressing metastatic breast cancer with trastuzumab (Herceptin®) and chemotherapy [abstract]. Breast Cancer Res Treat 2000 Nov; 64: 123
Burstein HJ, Harris LN, Kaelin CM, et al. Preoperative trastuzumab (T) and paclitaxel (P) for HER2-overexpressing (HER2+) stage II/III breast cancer [abstract no. 537]. Eur J Cancer 2001 Oct 21; 37 Suppl. 6: 146
Carey LA, Dees C, Sartor C, et al. Safety and efficacy of 4AC followed by paclitaxel plus trastuzumab in high risk breast cancer patients [abstract no. 1856]. Proc Am Soc Clin Oncol 2001 May 12; 20(2): 27
Hurley J, Franco S, Velez P, et al. Primary therapy with Herceptin, Taxotere and Cisplatin in locally advanced and inflammatory breast cancer [abstract no. 1871]. Proc Am Soc Clin Oncol 2001 May 12; 20 (2): San Francisco, CA, 31
Nelson NJ. Can HER2 status predict response to cancer therapy? J Natl Cancer Inst 2000 Mar 1; 92(5): 366–7
Tubbs RR, Pettay JD, Roche PC, et al. Discrepancies in clinical laboratory testing of eligibility for trastuzumab therapy: apparent immunohistochemical false-positives do not get the message. J Clin Oncol 2001 May; 19(10): 2714–21
Acosta G, Askaa J, Bilous M, et al. Initial report of the HER2000 study: a multinational study of HER2 status of breast cancer using immunohistochemistry (IHC; Herceptest) [abstract]. EurJ Cancer 2001 Oct 21; 37 Suppl. 6: S241
Mass RD, Press M, Anderson M, et al. Improved survival benefit from Herceptin (trastuzumab) in patients selected by fluorescence in situ hybridization (FISH) [abstract no. 85]. Proc Am Soc Clin Oncol 2001 May 12; 20: San Francisco, CA, 22
Cobleigh M, Vogel C, Tripathy D, et al. Fluorescence in situ hybridization (FISH) may accurately select patients likely to benefit from herceptin monotherapy [abstract]. Eur J Cancer 2001 Oct 21; 37 Suppl. 6: S192
Boettcher B, Kahlert S, Bauerfeind I, et al. Loss of HER-2 overexpression during Herceptin®-therapy in metastatic breast cancer [abstract]. Breast Cancer Res Treat 2000 Nov; 64: 109
Osoba D, Slamon DJ, Burchmore M, et al. Effects of treatment with Her2mab (trastuzumab/Herceptin) plus chemotherapy (H+C) versus chemotherapy alone (C) on health-related quality of life (HRQL) in women with HER-2/neu-overexpressing metastatic breast cancer [abstract no. 109]. Proc Am Soc Clin Oncol; 2001 May 12–15 San Francisco (CA); 20 (Pt 1): 28
Osoba D, Burchmore MJ, Ash M, et al. Health related quality of life (HRQL) of patients treated with first-line, single agent, trastuzumab (Herceptin) for metastatic Her-2 overexpressing breast cancer (MBC) [abstract]. 36th Proc Am Soc Clin Oncol 2000 May 20; 19: 436a
Lieberman G, Burchmore MJ, Ferhenbacher L, et al. Health related quality of life (HRQL) of patients with HER-2 overexpressing, metastatic breast cancer (MBC) treated with Herceptin (trastuzumab) as a single agent [abstract no. 1613]. Proc Am Soc Clin Oncol 1999 May 15; 18: 417a
Mermershtain W, Cohen AD, Geffen DB, et al. Cutaneous photosensitivity associated with abberations in the heme biosynthetic pathway induced by taxanes (T) and trastuzumab (H): a report of 3 cases of metastatic breast cancer. Ann Oncol 2000; 11 Suppl. 4: 28
Nissenblatt MJ, Karp GI. Bleeding risk with trastuzumab (Herceptin) treatment. JAMA 1999 Dec 22–29; 282: 2299–300
Behr TM, Béhé M. Trastuzumab and breast cancer [letter]. N Engl J Med 2001 Sep 27; 345(13): 995–6
Tripathy D, Scidman A, Hudis C, et al. Effect of cardiac dysfunction on treatment outcome in the herceptin pivotal trial [abstract]. Eur J Cancer 2001 Oct 21; 37 Suppl. 6: S193
Keefe DL, Spaltro B, Pierri MK. Cardiovascular adverse effects of trastuzumab in patients with stage IV breast cancer [abstract]. Clin Pharmacol Ther 2001 Feb; 69 Suppl.: 6
Theodoulou M, Campos S, Welles G, et al. Cardiac safety and efficacy of TLC D99 (D99) and trastuzumab in patients with advanced breast cancer [abstract no. 712]. Eur J Cancer 2001 Oct; 37 Suppl. 6: 195
Untch M, Schaller G, Jaenicke F, et al. Cardiac safety of herceptin® in combination with epirubicin plus cyclophosphamide: interim results of a phase II study in patients with metastatic breast cancer [abstract no. 724]. Eur J Cancer 2001 Oct 21; 37 Suppl. 6: S198
Gianni L, Eiermann W, Borquez D, et al. Cardiac safety and antitumor activity of doxorubicin and taxol followed by weekly taxol (AT & T) when herceptin is initiated with AT or with T alone in women with HER2-positive advanced breast cancer [abstract no. 698]. Eur J Cancer 2001 Oct 21; 37 Suppl. 6: S191
Genentech Inc. Important drug warning [letter]. May 3, 2000
Melamed J, Sajer S, Dubois J, et al. Rapid desensitization and rush immunotherapy to trastuzumab (Herceptin) [abstract no.37]. J Allergy Clin Immunol 2001 Feb; 107(2): S11
Horton J. HER2 and trastuzumab in breast cancer. Cancer Control 2001 Jan/Feb; 8(1): 103–10
Hortobagyi GN. Developments in chemotherapy of breast cancer. Cancer 2000 Jun 15; 88(12) Suppl.: 3073–9
Mass RD, Murphy M, Cobleigh M, et al. Relationship of estrogen receptor (ER) status to clinical benefit in clinical trials of herceptin [abstract]. Eur J Cancer 2001 Oct 21; 37 Suppl. 6: S190
Nelson NJ. Experts debate value of HER2 testing methods. J Natl Cancer Inst 2000 Feb 16; 92(4): 292–4
McNeil C. Herceptin in the adjuvant setting: phase III trials begin. J Natl Cancer Inst 2000 May 3; 92(9): 683–4
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: N. Bangemann, Medical Center Benjamin Franklin, Free University, Berlin, Germany; H. Burstein, Dana-Faber Cancer Institute, Boston, Massachussetts, USA; L. A. Carey, UNC Lineberger Comprehensive Cancer Center, University of North Carolina, Chapel Hill, North Carolina, USA; I.O. Ellis, Department of Histopathology, City Hospital, Nottingham, England; V. Harvey, Department of Clinical Oncology, Auckland Hospital, Auckland, New Zealand; J. Horton, H. Lee Moffitt Cancer Center & Research Institute, Tampa, Florida, USA; B. Leyland-Jones, Department of Oncology, McGill University, Montreal, Canada; R. Seshadri, Flinders Medical Centre, Bedford Park, South Australia, Australia.
Data Selection
Sources: Medical literature published in any language since 1983 on trastuzumab, identified using AdisBase (a proprietary database of Adis International), Medline and EMBASE. Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: AdisBase search terms were ‘trastuzumab’ or ‘anti-HER-2-monoclonal-antibody’ or ‘HER-2’. Medline search terms were ‘trastuzumab’ or ‘HER-2’. EMBASE search terms were ‘trastuzumab’ or ‘HER-2’. Searches were last updated 28 Nov 2001.
Selection: Studies in patients with metastatic breast cancer overexpressing HER2 who received trastuzumab. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Metastatic breast cancer, monoclonal antibody, pharmacodynamics, pharmacokinetics, therapeutic use.
Rights and permissions
About this article
Cite this article
McKeage, K., Perry, C.M. Trastuzumab. Drugs 62, 209–243 (2002). https://doi.org/10.2165/00003495-200262010-00008
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200262010-00008