Abstract
Macromolecular drugs (also referred to as polymeric drugs) are a diverse group of drugs including polymer-conjugated drugs, polymeric micelles, liposomal drugs and solid phase depot formulations of various agents. In this review we will consider only water-soluble macromolecular drugs. In common, such drugs have high molecular weights, more than 40 kDa, which enables them to overcome renal excretion. Consequently, this group of drugs can attain prolonged plasma or local half-lives.
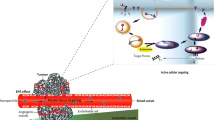
The prolonged circulating time of these macromolecules enables them to utilise the vascular abnormalities of solid tumour tissues, a phenomenon called the enhanced permeability and retention (EPR) effect. The EPR effect facilitates extravasation of polymeric drugs more selectively at tumour tissues, and this selective targeting to solid tumour tissues may lead to superior therapeutic benefits with fewer systemic adverse effects. This contrasts with conventional low-molecular-weight drugs, where intratumour concentration diminishes rapidly in parallel with plasma concentration. The EPR effect is also operative in inflammatory tissues, which justifies the development and use of this class of drugs in infectious and inflammatory conditions.
At the present time, several polymeric drugs have been approved by regulatory agencies. These include zinostatin stimalamer (copolymer styrene maleic acid-conjugated neocarzinostatin, or SMANCS) and polyethyleneglycol-conjugated interferon-α-2a. This article discusses these and other polymeric drugs in the setting of targeting to solid tumours.








Similar content being viewed by others
Notes
Use of tradenames is for product identification only and does not imply endorsement
References
World Health Organization. Press release WHO/52, 28 June 2002. Available online from: http://www.who.int/inf/en/pr-2002-52.html [Accessed 2003 Aug 23]
Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumor tropic accumulation of proteins and antitumor agent SMA-neocarzinostatin. Cancer Res 1986; 46: 6387–92
Noguchi Y, Wu J, Duncan R, et al. Early phase of tumor accumulation of macromolecules: a great difference in clearance rate between tumor and normal tissues. Jpn J Cancer Res 1998; 89: 307–14
Iwai K, Maeda H, Konno T. Use of oily contrast medium for selective drug targeting to tumor: enhanced therapeutic effect and X-ray image. Cancer Res 1984; 44: 2114–21
Folkman J. Tumor angiogenesis in cancer, therapeutic implications. N Engl J Med 1971; 285: 1182–6
Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995; 1: 27–31
Wu J, Akaike T, Maeda H. Modulation of enhanced vascular permeability in tumors by a bradykinin antagonist, a cyclooxygenase inhibitor, and a nitric oxide scavenger. Cancer Res 1998; 58: 159–65
Maeda H, Akaike T, Wu J, et al. Bradykinin and nitric oxide in infectious disease and cancer. Immunopharmacology 1996; 33: 222–30
Matsumura Y, Kimura M, Yamamoto T, et al. Involvement of the kinin-generating cascade and enhanced vascular permeability in tumor tissue. Jpn J Cancer Res 1988; 79: 1327–34
Maeda H, Matsumura Y, Kato H. Purification and identification of [hydroxypropyl 3] bradykinin in ascitic fluid from a patient with gastric cancer. Jpn J Cancer Res 1994; 85: 331–4
Doi K, Akaike T, Horie H, et al. Excessive production of nitric oxide in rat solid tumor and its implication in rapid tumor growth. Cancer 1996; 77: 1598–04
Doi K, Akaike T, Fujii S, et al. Induction of haem oxygenase-1 by nitric oxide and ischaemia in experimental solid tumors and implications for tumor growth. Br J Cancer 1999; 80: 1945–54
Senger DR, Galli SJ, Dvorak AM, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983; 219: 983–5
Wu J, Akaike T, Hyashida K, et al. Enhanced vascular permeability in solid tumor involving peroxynitrite and matrix metalloproteinases. Jpn J Cancer Res 2001; 92: 439–51
Arshady R, editor. Polymeric biomaterials, the PBM Series, Vol 1: introduction to polymeric biomaterials. London: Citus Books, 2003: 233–61
Maeda H, Kabanov A, Kataoka K, et al., editors. Polymer drugs in the clinical stage: advantages and prospects. New York: Kluwer Academic, 2003
Schechter B, Neumann A, Wilchek M, et al. Soluble polymers as carriers of cisplatinum. J Control Release 1989; 10: 75–87
Li C, Yu DF, Newman R, et al. Complete regression of well-established tumors using a novel water-soluble poly (L-glutamic acid)-paclitaxel conjugate. Cancer Res 1998; 58: 2404–9
Singer JW, De Vries P, Bhatt R, et al. Conjugation of camptothecins to poly-(L-glutamic acid). Ann N Y Acad Sci 2000; 922: 136–50
Al-Shamkhani A, Duncan R. Alginates as a macromolecular drug delivery system for the antitumor agent daunomycin: synthesis, controlled release properties and antitumor activity of alginate-cis-aconityl-daunomycin conjugates. Int J Pharm 1995; 122: 107–19
Ohya Y, Inosaka K, Ouchi T. Synthesis and antitumor activity of 6-O-carboxymethyl chitin fixing 5-fluorouracil through pentamethylene, monomethylene spacer groups via amide, ester bonds. Chem Pharm Bull (Tokyo) 1992; 40: 559–61
Yashima E, Uchida S, Akashi M, et al. Polymer drugs and polymeric drugs. Part VII. Antitumour conjugated polymeric drugs consisting of 5-fluorouracil and polyanionic polymers. J Bioact Compat Polym 1990; 5: 53–64
Danhauser-Riedl S, Hausmann E, Schick HD, et al. Phase I clinical and pharmacokinetic trial of dextran conjugated doxorubicin (AD-70, DOX-OXD). Invest New Drugs 1993; 11: 187–95
Munechika K, Sogame Y, Kishi N, et al. Tissue distribution of macromolecular conjugate adriamycin linked to oxidized dextran, in rat and mouse bearing tumor-cells. Biol Pharm Bull 1994; 17: 1193–8
Bernstein A, Hurwitz E, Arnon R. Higher antitumor efficacy of daunomycin when linked to dextran: in vivo and in vitro studies. J Natl Cancer Inst 1978; 60: 379–84
Takakura Y, Atsumi R, Hashida M, et al. Development of a novel polymeric prodrug of mitomycin C, mitomycin C-dextran conjugate with anionic charge: II. disposition and pharmacokinetics following intravenous and intramuscular administration. Int J Pharm 1987; 37: 145–54
Takakura Y, Kitajima M, Matsumoto S, et al. Development of a novel polymeric prodrug of mitomycin C, mitomycin C-dextran conjugate with anionic charge: I. physicochemical characteristics and in vivo and in vitro antitumour activities. Int J Pharm 1987; 37: 135–43
Okuno S, Harada M, Yano T, et al. Complete regression of xenografted human carcinomas by camptothecin analoguecarboxymethyl dextran conjugate (T-0128). Cancer Res 2000; 60: 2988–95
Schechter B, Pauzner R, Arnon R, et al. Cisplatinum (II) complexes of carboxymethyl dextran as potential antitumor agents: I. Preparation and characterization. Cancer Biochem Biophys 1986; 8: 277–87
Ichinose K, Tomiyama N, Nakashima M, et al. Antitumor activity of dextran derivatives immobilizing platinum complex (II). Anticancer Drags 2000; 11: 33–8
Nogusa H, Yano T, Okuno S, et al. Synthesis of carboxymethylpullulan-peptide-doxorabicin conjugates and their properties. Chem Pharm Bull 1995; 43: 1931–6
Nogusa H, Hamana H, Uchida N, et al. Improved in vivo antitumor efficacy and reduced systemic toxicity of carboxymethylpullulan-peptide-doxorabicin conjugates. Jpn J Cancer Res 2000; 91: 1333–8
Hirano T, Ringsdorf H, Zaharko D. Antitumor activity of monomeric and polymeric cyclophosphamide derivative compound with in vitro hydrolysis. Cancer Res 1980; 40: 2263–7
Pratesi G, Tortoreto M, Zunino F. Increased effect of doxorubicin linked to pyran copolymer in the intracavity treatment of human ovarian carcinoma in nude mice. Reg Cancer Treat 1990; 3: 40–3
Duncan R, Hume IC, Yardley HJ, et al. Macromolecular prodrugs for use in targeted cancer chemotherapy: melphalan covalently coupled to N-(2-hydroxypropyl) methacrylamide copolymers. J Control Release 1991; 16: 121–36
Putnam DA, Kopecek J. Enantioselective release of 5-fluorouracil from N-(2-hydroxypropyl) methacrylamide-based copolymers via lysosomal enzymes. Bioconjug Chem 1995; 6: 483–92
Vasey P, Kaye S, Morrison R, et al. Phase I clinical and pharmacokinetic study of PKI (HPMA copolymer doxorabicin): first member of a new class of chemotherapeutic agents-drag-polymer conjugates. Clin Cancer Res 1999; 5: 83–94
Kopecek J, Kopeckova P, Minko T, et al. HPMA copolymeranticancer drug conjugates: design, activity, and mechanism of action. Eur J Pharm Biopharm 2000; 50: 61–81
Caiolfa VR, Zamai M, Fiorino A, et al. Polymer-bound camptothecin: initial biodistribution and antitumor activity studies. J Control Rel 2000; 65: 105–19
Greenwald RB, Gilbert CW, Pendri A, et al. Drag delivery systems: water soluble taxol 2′-poly(ethylene glycol) ester prodrugs -design and in vivo effectiveness. J Med Chem 1996; 39: 424–31
Li C, Yu D, Inoue T, et al. Synthesis and evaluation of watersoluble polyethylene glycol-paclitaxel conjugate as a paclitaxel prodrag. Anticancer Drags 1996; 7: 642–8
Pendri A, Conover CD, Greenwald RB. Antitumor activity of paclitaxel-2′-glycinate conjugated to poly (ethylene glycol): a water-soluble prodrag. Anticancer Drag Des 1998; 13: 387–95
Zhang X, Burt HM, Mangold G, et al. Anti-tumor efficacy and biodistribution of intravenous polymeric micellar paclitaxel. Anticancer Drags 1997; 8: 696–01
Ramaswamy M, Zhang X, Burt HM, et al. Human plasma distribution of free paclitaxel and paclitaxel associated with diblock copolymers. J Pharm Sci 1997; 86: 460–4
Zhang X, Burt HM, Von Hoff D, et al. An investigation of the antitumor activity and biodistribution of polymeric micellar paclitaxel. Cancer Chemother Pharmacol 1997; 40: 81–6
Mizumura Y, Matsumura Y, Hamaguchi T, et al. Cisplatinincorporated polymeric micelles eliminate nephrotoxicity, while maintaining antitumor activity. Jpn J Cancer Res 2001; 92: 328–36
Yokoyama M, Miyauchi M, Yamada N, et al. Characterization and antitumor activity of the micelle-forming polymeric anticancer drag doxorubicin-conjugated poly (ethylene glycol)-poly (aspartic acid) block copolymer. Cancer Res 1990; 50: 1693–00
Yokoyama M, Okano T, Sakurai Y, et al. Toxicity and antitumor activity against solid tumors of micelle-forming polymeric anticancer drug and its extremely long circulation in blood. Cancer Res 1991; 51: 3229–36
Yokoyama M, Okano T, Sakurai Y, et al. Selective delivery of adriamycin to a solid tumor using a polymeric micelle carrier system. J Drag Target 1999; 7: 171–86
Merdan T, Kopecek J, Kissel T. Prospects for cationic polymers in gene and oligonucleotide therapy against cancer. Adv Drug Deliv Rev 2002 Sep 13; 54: 715–58
Fischer D, Li Y, Ahlemeyer B, et al. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials 2003; 24: 1121–31
Kopecek J, Kopeckova P, Minko T, et al. Water-soluble polymers in tumor targeted delivery. J Control Release 2001 Jul 6; 74: 147–58
Gabizon AA. Pegylated liposomal doxorubicin: metamorphosis of an old drug into a new form of chemotherapy. Cancer Invest 2001; 19: 424–36
Brocchini S, Duncan R. Pendent drugs, release from polymers. In: Mathiowitz E, editor. Encyclopedia of controlled drug delivery. New York: John Wiley & Sons Inc, 1999: 786–816
Sawa T, Sahoo SK, Maeda H. Water-soluble polymer in medicine. In: Arshady R, editor. Polymeric biomaterials, The PBM Series, Vol 1: introduction to polymeric biomaterials. London: Citus Books, 2003: 233–61
Maeda H, Matsumura Y, Oda T, et al. Cancer selective macromolecular therapeutics: tailoring of an antitumor protein drug. In: Feeny RE, Whitaker JR, editors. Protein tailoring for food and medical uses. New York: Marcel Dekker Inc, 1986: 353–82
Maeda H, Takeshita J, Kimura M. Improvement of pharmacological properties of protein drags by tailoring with synthetic polymers. J Bioact Compat Polym 1988; 3: 27–43
Bartholeyns J, Moore S. Pancreatic ribonuclease: enzymatic and physiological properties of cross-linked dimer. Science 1974; 186: 444–5
Takakura T, Kaneko Y, Hashida M, et al. Control of pharmaceutical properties of soybean trypsin inhibitor by conjugation with dextran: I. synthesis and characterization. J Pharm Sci 1989; 78: 117–21
Takakura T, Fujita T, Hashida M, et al. Control of pharmaceutical properties of soybean trypsin inhibitor by conjugation with dextran II. Biopharmaceutical and pharmacological properties. J Pharm Sci 1989; 78: 219–22
Ogino T, Inoue M, Ando Y, et al. Chemical modification of Superoxide dismutase: extension of plasma half-life of the enzyme through its reversible binding to the circulating albumin. Int J Pept Protein Res 1988; 32: 153–9
Kimura M, Matsumora Y, Miyauchi Y, et al. A new tactic for the treatment of jaundice: an injectable polymer-conjugated bilirubin oxidase. Pro Soc Exp Biol Med 1988; 188: 364–9
Buys CHCM, Dejong ASH, Bouma JDW, et al. Rapid uptake by liver sinusoidal cells of serum albumin modified with retention of its compact conformation. Biochim Biophys Acta 1975; 392: 95–100
Kamisaki Y, Wada H, Yagura H, et al. Reduction in immunogenicity and clearance rate of Escherichia coli L-asparaginase by modification with monomethoxy polyethylene glycol. J Pharmacol Exp Ther 1981; 216: 410–4
Ney K, Gidwitz S, Pizzo S. Binding and endocytosis of α2-nacroglobulin-plasmin complex. Biochemistry 1985; 24: 4586–92
Wills RJ. Clinical pharmacokinetics of interferons. Clin Pharmacokinet 1990; 19: 390–9
Glue P, Fang J, Sabo WS, et al. Pegylated interferon-α2b: pharmacokinetics, pharmaco-dynamics, safety, and preliminary efficacy data. Clin Pharmacol Ther 2000; 68: 556–67
Heathcote EJ, Shiftman ML, Coooksley WG, et al. PEG-interferon alpha-2a in patients with chronic hepatitis C and cirrhosis. N Engl J Med 2000; 343: 1673–80
Duncan R, Rejmanova P, Kopecek J, et al. Pinocytic uptake and intracellular degradation of N-(2-hydroxypropyl) methacrylamide copolymers: a potential drug delivery system. Biochim Biophys Acta 1981; 678: 143–50
Maeda H, Aikawa S, Yamashita A. Subcellular fate of protein antibiotic neocarzinostatin in culture of lymphoid cell line from Burkett’s lymphoma. Cancer Res 1975; 35: 554–9
Oda T, Maeda H. Binding to and internalization by cultured cells of neocarzinostatin and enhancement of its action by conjugation with lipophilic styrene-maleic acid copolymer. Cancer Res 1987; 47: 3206–11
Pellegrin P, Fernandez A, Lamb NJ, et al. Macromolecular uptake is a spontaneous event during mitosis in cultured fibroblasts: implications for vector-dependent plasmid transfection. Mol Biol Cell 2002; 13: 570–8
Miyamoto Y, Oda T, Maeda H. Comparison of the cytotoxic effects of the high- and low-molecular-weight anticancer agents on multidrug-resistant Chinese hamster ovary cells in vitro. Cancer Res 1990; 150: 1571–5
St’astny M, Strohalm J, Plocova D, et al. Possibility to overcome P-glycoprotein (PGP)-mediated multidrug resistance by antibody-targeted drugs conjugated to N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer carrier. Eur J Cancer 1999; 35: 459–66
Folkman J. What is the evidence that tumors are angiogenesis dependent?. J Natl Cancer Inst 1990 Jan 3; 82: 4–6
Maeda H, Sawa T, Konno T. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J Control Release 2001; 74: 47–61
Maeda H. Enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul 2001; 41: 189–07
Maeda H, Fang J, Inuzuka T, et al. Vascular permeability enhancement in solid tumors: various factors, mechanisms involved and its implications. Int Immunopharmacol 2003; 3: 319–28
Jain R. Barriers to drug delivery in solid tumors. Sci Am 1994; 271: 58–65
Seymour LW, Miyamoto Y, Maeda H, et al. Influence of molecular weight on passive tumor accumulation of a soluble macromolecular drug carrier. Eur J Cancer 1995; 31A: 766–70
Duncan R. Polymer conjugates for tumor targeting and intercytoplasmic delivery: the EPR effect as a common gateaway?. Pharm Sci Technol Today 1999; 2(11): 441–9
Yuan F, Dellian M, Fukumura D, et al. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res 1995; 55: 3752–6
Hashizume H, Baluk P, Morikawa S, et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol 2000; 156: 1363–80
Ohkouchi K, Imoto H, Takakura Y, et al. Disposition of anticancer drugs after bolus arterial administration in a tissueisolated tumor perfusion system. Cancer Res 1990; 50: 4396–401
Konno T, Maeda H, Iwai K, et al. Selective targeting of anticancer drug and simultaneous image enhancement in solid tumors by arterially administered lipid contrast medium. Cancer 1984; 54: 2367–74
Maeda H. SMANCS and polymer-conjugated macromolecular drugs: advantages in cancer chemotherapy. Adv Drug Deliv Rev 1991; 6: 181–02
Iwai K, Maeda H, Konno T, et al. Tumor targeting by arterial administration of lipids: rabbit model with VX2 carcinoma in the liver. Anticancer Res 1987; 7: 321–7
Marecos E, Weissleder R, Bogdanov A. Antibody-mediated versus nontargeted delivery in a human small cell lung carcinoma model. Bioconjug Chem 1998; 9: 184–91
Skinner S, Tutton P, O’Brien PE. Microvascular architecture of experimental colon tumors in the rat. Cancer Res 1990; 50: 2411–7
Suzuki M, Takahashi T, Sato T. Medial regression and its functional significance in tumor supplying host arteries. Cancer 1987; 59: 444–50
Li CJ, Miyamoto Y, Kojima Y, et al. Augmentation of tumour delivery of macromolecular drugs with reduced bone marrow delivery by elevating blood pressure. Br J Cancer 1993; 67: 975–80
Maeda H, Wu J, Okamoto T, et al. Kallikrein-kinin in infection and cancer. Immunopharmacology 1999; 43: 115–28
Suzuki M, Hori K, Abe Z, et al. A new approach to cancer chemotherapy: selective enhancement of tumor blood flow with angiotensin II. J Natl Cancer Inst 1981; 67: 663–9
Hori K, Saito S, Takahashi H, et al. Tumor-selective blood flow decrease induced by an angiotensin converting enzyme inhibitor, temocapril hydrochloride. Jpn J Cancer Res 2000; 91: 261–9
Hori K, Suzuki M, Tanda S, et al. Fluctuation in tumor blood flow under normotension and the effect of angiotensin-II induced hypertension. Jpn J Cancer Res 1991; 82: 1309–16
Wu J, Akaike T, Hayashida K, et al. Identification of bradykinin receptors in clinical cancer specimens and murine tumor tissues. Int J Cancer 2002; 98: 29–35
Kamata R, Yamamoto T, Matsumoto K, et al. A serratial protease causes vascular permeability reaction by activation of the Hageman factor-dependent pathway in guinea pig. Infect Immun 1985; 48: 747–53
Tanaka S, Akaike T, Wu J, et al. Tumor selective vascular blood flow modulation accompanying extravasation induced by stable prostaglandin I2analogue, beraprost sodium. J Drug Target 2003; 11: 45–52
Yamaguchi T, Takahashi T, Kitamura K, et al. Application of tumor marker for immunotargeting therapy of cancer. Nippon Rinsho 1996; 54: 1674–9
Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer 2002; 2: 750–63
Ranson M, Sliwkowski MX. Perspectives on anti-HER monoclonal antibodies. Oncology 2002; 63 Suppl. 1: 17–24
King KM, Younes A. Rituximab: review and clinical applications focusing on non-Hodgkin’s lymphoma. Expert Rev Anticancer Ther 2001; 1: 177–86
Maeda H. Neocarzinostatin in cancer chemotherapy (review). Anticancer Res 1981; 1: 175–86
Edo K, Koide Y. Neocarzinostatin chromophore: structure and mechanism of DNA cleavage. In: Maeda H, Edo K, Ishida N, editors. Neocarzinostatin: the past, present, and future of an anticancer drug. Tokyo: Springer-Verlag, 1997: 23–45
Maeda H, Ueda M, Morinaga T, et al. Conjugation of poly(styrene-co-maleic acid) derivatives to the antitumor protein neocarzinostatin: pronounced improvements in pharmacological properties. J Med Chem 1985; 28: 455–61
Oda T, Morinaga T, Maeda H. Stimulation of macrophage by polyanions and its conjugated proteins and effect on cell membrane. Proc Soc Exp Biol Med 1986; 181: 9–17
Oda T, Sato F, Maeda H. Facilitated internalization of neocarzinostatin and its lipophilic polymer conjugate, SMANCS, into cytosol in acidic pH. J Natl Cancer Inst 1987; 79: 1205–11
Oka K, Miyamoto Y, Matsumura Y, et al. Enhanced intestinal absorption of a hydrophobic polymer-conjugated protein drug, SMANCS, in an oily formulation. Pharm Res 1990; 7: 852–5
Kobayashi A, Oda T, Maeda H. Protein binding of macromolecular anticancer agent SMANCS: characterization of poly (styrene-co-maleic acid) derivatives as an albumin binding ligand. J Bioact Compat Polym 1988; 3: 319–33
Maeda H, Matsumoto T, Konno T, et al. Tailor-making of protein drugs by polymer conjugation for tumor targeting: a brief review on SMANCS. J Protein Chem 1984; 3: 181–93
Konno T, Maeda H, Iwai K, et al. Effect of arterial administration of high-molecular weight anticancer agent SMANCS with lipid lymphographic agent on hepatoma: a preliminary report. Eur J Cancer Clin Oncol 1983; 19: 1053–65
Maki S, Konno T, Maeda H. Image enhancement in computerized tomography for sensitive diagnosis of liver cancer and semiquantitation of tumor selective drug targeting with oily contrast medium. Cancer 1985; 56: 751–7
Konno T, Kai Y, Yamashita R, et al. Targeted chemotherapy for unresectable primary and metastatic liver cancer. Acta Oncol 1994; 33: 133–7
Seymour LW, Olliff SP, Poole CJ, et al. A novel dosage approach for evaluation of SMANCS in the treatment of primary hepatocellular carcinoma. Int J Oncol 1998; 12: 1217–23
Reddy K, Wright T, Pockros P, et al. Efficacy and safety of pegylated (40-kDa) interferon alpha-2a compared with interferon alpha-2a in noncirrhotic patients with chronic hepatitis C. Hepatology 2001; 33: 433–8
Karasiewicz R, Nalin C, Rosen P. PEG-interferon conjugates, compound no. (Ro25-2854, Ro24-9485). US Patent 5,382,657. 1995 Jan 17
Rajender R, Modi M, Pedder S. Use of peginterferon α-2a (40KD) (Pegasys®) for the treatment of hepatitis C. Adv Drug Deliv Rev 2002; 54: 571–86
Fried M, Shiffman M, Reddy K, et al. Peginterferon α-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002; 347: 975–82
Zeuzem S, Feinman SV, Rasenack J, et al. PEG-interferon alfa-2a in patients with chronic hepatitis C. N Engl J Med 2000; 343: 1666–772
Skubitz KM. Phase II trial of pegylated-liposomal doxorubicin (Doxil) in mesothelioma. Cancer Invest 2002; 20: 859–60
Matsumora Y. An interim analysis of phase I clinical trial of MCC-456, doxorubicin encapsulated in PEG immunoliposome, in patients with metastatic stomach cancer. In: Maeda H, Kabanov A, Kataoka K, et al. editors. Polymer drugs in the clinical stage: advantages and prospects. New York: Kluwer Academic, 2003: 179–94
Hershfield MS, Buckley R, Greenberg M, et al. Treatment of adenosine deaminase deficiency with polyethylene glycolmodified adenosine deaminase. N Engl J Med 1987; 310: 586–9
Abuchowski A, Kazo G, Verhowest J, et al. Cancer therapy with chemically modified enzymes: I. antitumor properties of polyethylene glycol-asparaginase conjugates. Cancer Biochem Biophys 1984; 7: 175–86
Acknowledgements
As the authors, we would like to acknowledge our colleagues William Regalson, Toshimitsu Konno, Helmet Ringsdorf, Ruth Duncan, Shojiro Maki and Tomohiro Sawa, for their enthusiasm, collaboration and support of our work in various aspects of the subject matter. We would also like to express our thanks to Citus Books for granting the permission to reproduce figure 1 of this review. Hiroshi Maeda acknowledges the continuous support for cancer grant in aid from the Ministry of Education, Science, Culture and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Greish, K., Fang, J., Inutsuka, T. et al. Macromolecular Therapeutics. Clin Pharmacokinet 42, 1089–1105 (2003). https://doi.org/10.2165/00003088-200342130-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200342130-00002