Summary
Molsidomine is a prodrug for the formation of nitric oxide (NO). Its pharmacokinetics are characterised by rapid absorption and hydrolysis, taking a short time to achieve maximal systemic concentrations of both the parent compound and its active metabolite, SIN-1. The time to peak plasma drug concentration (tmax) is 1 to 2 hours. The bioavailability of the parent compound after oral administration in tablet form is 44 to 59%, but further metabolism to release NO and form polar metabolites is rapid; the half-life (t½) of SIN-1 is 1 to 2 hours. Urinary excretion accounts for more than 90% of the part of the administered dose of molsidomine which is not excreted unchanged. Protein binding of the parent compound is very low (3 to 11 %) and its volume of distribution (Vd) corresponds to the range of body weight.
Single-dose studies (1,2 and 4mg) have revealed linear pharmacokinetics, and multiple dose studies in healthy individuals (2mg 3 times daily for 7 days) and coronary artery disease (CAD) patients (4mg 4 times daily for 4 weeks) do not show any accumulation of the drug.
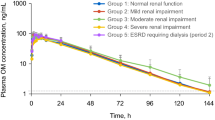
A study in young and elderly individuals indicated that the first-pass effect is decreased and t½ prolonged with age, resulting in an increased area under the concentration-time curve (AUC) of molsidomine and SIN-1. In patients with liver disease and congestive heart failure similar changes were observed, but much less so in patients with CAD. Clearance was also impaired in patients with liver disease, but the pharmacokinetics of molsidomine were not markedly altered by impaired renal function. In general, due to a large therapeutic dose range, dosage adjustments are not required on the basis of clinical experience. In certain patients a lower starting dose may be recommended, such as in those with impaired liver or kidney function, in congestive heart failure or in the presence of concomitant treatment with other vasoactive compounds.
A linear dose-effect relationship is observed with counterclockwise hysteresis, i.e. a greater effect associated with the decrease of plasma concentrations than during their increase, which may be at least partly due to the metabolic delay in the formation of NO from SIN-1. Accordingly, the duration of action of molsidomine is longer than would be expected on the basis of the elimination half-life.
The pharmacokinetics of molsidomine support the recommended dosages for use in angina pectoris.
Similar content being viewed by others
References
Hashimoto K, Taira N, Hirata M, et al. The mode of hypotensive action of newly synthesized sydnonimine derivatives. Drug Res 1971; 21: 1329–32
Ignarro LJ, Buga GM, Wood KS, et al. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA 1987; 84: 9265–9
Anderson TJ, Meredith IT, Ganz P, et al. Nitric oxide and nitrovasodilators: similarities, differences and potential interactions. J Am Coll Cardiol 1994; 24: 555–66
Majid PA, De Feyter PJ, Van der Wall EE, et al. Molsidomine in the treatment of patients with angina pectoris: acute hemodynamic effects and clinical efficacy. N Engl J Med 1980; 302: 1–6
Vogt A, Kreuzer H. Comparative study of the hemodynamic effects of oral molsidomine and isosorbide dinitrate in man. Eur J Clin Pharmacol 1982; 23: 11–4
Unger P, Vachiery J-L, de Cannière D, et al. Comparison of the hemodynamic responses to molsidomine and isosorbide dinitrate in congestive heart failure. Am Heart J 1994; 128: 557–63
Tanayama S, Fujita T, Shirakawa Y, et al. Metabolic fate of 5-ethoxycarbonyl-3-morpholino-sydnonimine (SIN-10): I. Absorption, excretion and tissue distribution in rats and mice. Jpn J Pharmacol 1970; 20: 413–23
Tanayama S, Nakai Y, Fujita T, et al. Biotransformation of molsidomine (N-ethoxycarbonyl-3-morpholino-sydnonimine), a new anti-anginal agent, in rats. Xenobiotica 1974; 4: 175
Ibrahim TM, Unger P, Sobolski J, et al. Hemodynamic effects of SIN-1 in acute left heart failure. Cardiovasc Drug Ther 1989; 3: 557–61
Wilson ID, Fromson JM, Illing HPA, et al. The metabolism of [l4C]N-ethoxycarbonyl-3-morpholinosydnonimine (molsidomine) in man. Xenobiotica 1987; 17: 93–104
Dell D, Fromson JM, Illing HPA, et al. Pharmacokinetics and pharmacodynamics of molsidomine in man. Br J Clin Pharmacol 1978; 5: 395–60
Data on file, Hoechst AG
Dell D, Chamberlain J. Determination of molsidomine in plasma by high-performance liquid column chromatography. J Chromatogr 1978; 146: 465–72
Singlas E, Martre H. Pharmacocinétique humaine de la molsidomine. Ann Cardiol Angiol 1983; 32: 503–9
Bergstrand R, Vedin A, Wilhelmsson C, et al. Intravenous and oral administration of molsidomine, a pharmacodynamic and pharmacokinetic study. Eur J Clin Pharmacol 1984; 27: 203–8
Spreux-Varoquax O, Ulmer B, Cordonnier P, et al. Pharmacokinetics of molsidomine and its active metabolite, SIN-1 (or linsidomine), in the elderly. Fundam Clin Pharmacol 1991; 5: 549–56
Wildgrube HJ, Ostrowski J, Chamberlain J, et al. Liver function and pharmacokinetics of molsidomine and its metabolite 3-morpholinosydnonimine in healthy volunteers. Arzneimittelforschung 1986; 7: 1129–33
Meinertz T, Brandstätter A, Trenk D, et al. Relationship between pharmacokinetics and pharmacodynamics of molsidomine and its metabolites in humans. Am Heart J 1985; 109: 644–8
Oltmanns D, Friedmann W, Ostrowski J. Zur Pharmakokinetik von Molsidomin bei hochdosierter Langzeitbehandlung des frischen Herzinfarkts. Herzmedizin 1982; 5: 3–13
Weiser JR, Heger KH, Oltmanns D, et al. Zur Pharmakokinetik von Molsidomin bei eingeschränkter Leberfunktion. Herzmedizin 1986; 9: 41–6
Strasser R, Klepzig H, Ostrowski J, et al. Molsidomin bei koronarer Herzkrankheit. Munch Med Wochenschr 1983; 125: 156–8
Ostrowski J, Schweizer P, Erbel R, et al. Correlation of pharmacokinetic data to clinical effect of molsidomine. Proceedings of the First European Congress on Biopharmacology and Pharmacokinetics: 1981 Apr 1–3; Clermont Ferrand 1981; 3: 418–424
Grosse-Heitmeyer W, Huber T, Grewe R. Vergleich von oraler und intravenöser Anwendung von Molsidomin bei Patienten mit Herzinsuffizienz. Med Klin 1994; 89: 54–7
Spreux-Varoquax O, Doll J, Dutot C, et al. Pharmacokinetics of molsidomine and its active metabolites, linsidomine, in patients with liver cirrhosis. Br J Clin Pharmacol 1991; 32: 399–401
Huber T, Grosse-Heitmeyer W, Rietbrock S, et al. Pharmacokinetics and pharmacodynamics of molsidomine in patients with liver dysfunction due to congestive heart failure. Int J Clin Pharmacol Ther Toxicol 1992; 30: 491–2
Palmer RMJ, Ferrige AG, Moncada S. Release of nitric oxide accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987; 327: 524–6
Stamler JS, Simon DI, Osborne JA, et al. S Nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci USA 1992; 89: 444–8
Hueppe D, Jaeger D, Tromm A, et al. Dosisabhängige Akutwirkung und Langzeiteinfluβ von Molsidomin auf die portale und kardiale Hämodynamik bei Patienten mit Leberzirrhose. Med Klin 1994; 89 Suppl. II: 65–8
Sennesael J, Verbeelen D, Degrés S, et al. Pharmacokinetics of linsidomine (SIN1) after single and multiple intravenous short infusions in patients with renal insufficiency. Int J Clin Pharmacol Toxicol 1993; 11: 533–41
Lehmann KH. Die Wirkung von Molsidomin auf das Bela-stungs-EKG in Abhängigkeit von der Blutplasmakonzentration des Pharmakons [dissertation]. Ulm: Fakultät für klinische Medizin der Universität Ulm, 1979
Brack MJ, More RS, Hubner PJ, et al. The effects of different nitrate preparations on plasma heparin concentrations and the activated thromboplastin time. Postgrad Med J 1994; 70: 100–3
Unger P, Leone A, Staroukine M, et al. Hemodynamic responses to molsidomine in patients with ischemic cardiomyopathy tolerant to isosorbide dinitrate. J Cardiovasc Pharmacol 1991; 18: 888–94
Abrams J. The role of nitrates in coronary heart disease. Arch Intern Med 1995; 155: 357–64
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rosenkranz, B., Winkelmann, B.R. & Parnham, M.J. Clinical Pharmacokinetics of Molsidomine. Clin-Pharmacokinet 30, 372–384 (1996). https://doi.org/10.2165/00003088-199630050-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-199630050-00004