Summary
This review deals with the pharmacokinetics of dextrans and hydroxyethylstarch, the most commonly used plasma expanders. The complex composition of these colloidal agents (broad range of molecular weight distribution in vitro and in vivo, ) confounds their specific assay and meaningful pharmacokinetic analysis. In addition, the time-dependent decline of plasma concentrations of the plasma expanders is at least biphasic, and in some clinical studies the time period for plasma concentration monitoring has been inadequate to characterise the terminal elimination phase.
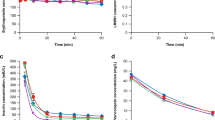
According to their average molecular weight, dextrans can be differentiated into dextran 1, dextran 40, dextran 60 and dextran 70. Metabolism of dextrans by dextranases and extrarenal excretion account for only 2 to 10% of the overall drug loss from the body. Persistence of dextrans in the systemic circulation and elimination by the renal route are dependent on the size of dextrans and their molecular weight distribution. Dextran species with a molecular weight below 15,000 daltons are filtered unrestricted, and consequently the elimination half-life of dextran 1 is relatively short (2 hours) and that of dextran 40 (10 hours) or dextran 60 (42 hours) much longer. In patients with renal insufficiency elimination is impaired in parallel to the reduction in glomerular filtration rate, and smaller doses are advisable in these patients. Dosage reduction might be also indicated if multiple infusions of dextrans are used, since dextran 40 accumulates considerably during long term use (particularly the fractions with higher molecular weights). As only about 50 to 70% of a single dose could be recovered within 48 hours in the urine, the remainder of the dose is probably stored somewhere in the body.
Disposition of hydroxyethylstarch is dependent on 2 major factors. As with dextrans, the molecular weight distribution affects the rate of renal elimination. In addition, the degree of substitution with hydroxyethyl groups mainly determines the metabolism of hydroxyethylstarch by α-amylase, and thus the overall elimination rate. A higher molecular weight range (e.g. hydroxyethylstarch 450,000 vs 200,000) and a more extensive degree of substitution (e.g. 0.7 vs 0.5) result in a slower elimination, as can be seen by comparing the half-life values of hydroxyethylstarch 450/0.7 (48 days) and hydroxyethylstarch 200/0.5 (20 days). Since only 40 to 65% of an infused dose could be recovered in the urine in humans, the remainder of the dose may be stored in the body. Animal experiments suggest that certain fractions of hydroxyethylstarch might be stored in some tissues. However, during multiple infusions with hydroxyethylstarch 200/0.5 for 10 days no accumulation was observed in the plasma of patients.
In conclusion, the disposition and pharmacological effects of plasma expanders are related to time-dependent changes in the molecular weight distribution of the plasma concentration decline. Unfortunately, the analytical assays applied in most studies were not able to differentiate the complex mixture of the infused colloids.
Similar content being viewed by others
References
Åberg B, Bloom WL, Hannson E. Gastrointestinal excretion of dextran C14. Acta Physiologica Scandinavica 52: 188–194, 1961
Ammon R. Das Vorkommen von Dextranase im menschlischen Gewebe. Enzymologia 25: 245–251, 1963
Appel W, Wirner V, Sprengard D. Quantitative Mikrobestimmung von Dextran 1. Bestimmung in Körperflössigkeiten. Zeitschrift für Klinische Chemie und Klinische Biochemie 6: 452–458, 1968
Arturson G, Granath K, Grotte G. Intravascular persistence and renal clearance of dextran of different molecular sizes in normal children. Archives of Diseases in Childhood 41: 168–171, 1966
Arturson G, Granath K, Thoren L, Wallenius G. The renal excretion of low molecular weight dextran. Acta Chirurgica Scandinavica 127: 543–551, 1964
Arturson G, Wallenius G. The intravascular persistence of dextran of different molecular sizes in normal humans. Scandinavian Journal of Clinical and Laboratory Investigation: 76–80, 1964
Boon JC, Jesch F, Ring J, Messmer K. Intravascular persistence of hydroxyethyl starch in man. European Journal of Surgical Research 8: 497–503, 1976
Burt JR. Automated analysis of sugar phosphates. Analytical Biochemistry 9: 293–302, 1964
Cullen MP, Turner C, Haycock GB. Gel permeation chromatography with interferometric refractometry for the rapid assay of polydisperse dextrans in biological fluids. Journal of Chromatography 337: 29–36, 1985
Emmrich P, Baumann W, Stechele U. Studies on the kinetics and renal excretion of low and high molecular weight dextrans in preterm babies, newborns and young infants. European Journal of Pediatrics 125: 181–190, 1977
Ferber HP, Nitsch E, Förster H. Studies on hydroxyethylstarch. Part 2: changes of molecular weight distribution for hydroxyethyl starch types 450/0.3,300/0.4, 200/0.7, 200/0.5, 200/0.3, 200/0.1 after infusion in serum and urine of volunteers. Arzneimittel-Forschung 35: 615–622, 1985
Förster H, Wicarkzyk C, Dudziak R. Bestimmung der Plasmaelimination von Hydroxyäthylstärke mittels verbesserter analytischer Methodik. Infusionstherapie 2: 88–92, 1981
Granath KA, Kvist BE. Molecular weight distribution analysis by gel chromatography on Sephadex. Journal of Chromatography 28: 69–81, 1967
Gray I. Metabolism of plasma expanders studied with carbon-14-labelled dextran. American Journal of Physiology 174: 462–466, 1953
Hulse J, Gibson TP, Look Z, McEntegart C, Yacobi A. Pharmacokinetics of hetastarch in patients with renal impairment. Clinical Pharmacology and Therapeutics 33: 254, 1983
Hulse JD, Yacobi A. Hetastarch: an overview of the colloid and its metabolism. Drug Intelligence and Clinical Pharmacy 17: 334–341, 1983
Jesch F, Hübner G, Zumtobel V, Zimmerman M, Messmer K. Hydroxyaethylstaerke (HAES 450/0.7) in Plasma und Leber: Konzentrations-verlauf und histologische Veränderungen beim Menschen. Infusionstherapie und Klinische Ernährung 6: 112–117, 1979
Köhler H, Kirch W, Höffler D, Köppe P. Pharmakokinetik und Dosierung von Dextran 40 in Abhängigkeit von der Nierenfunktion. Klinische Wochenschrift 52: 1111–1116, 1974
Köhler H, Kirch W, Klein H, Distler A. Plasmavolumen bei Dialysepatienten nach Infusion von 500ml 6% Hydroxyäthylstärke 450/0.7, 10% Dextran 40 bzw 3.5% isocyanatvernetzter Gelatine. Verhandlungen der Deutschen Gesellschaft für Innere Medizin 84: 1236–1238, 1979
Köhler H, Kirch W, Vogt J, Höffler D. Pharmakokinetik von Hydroxyäthylstärke bei Niereninsuffizienz. Verhandlungen der Deutschen Gesellschaft für Innere Medizin 83: 1676–1678, 1977
Köhler H, Weihrauch TR, Fiegel P, Kirch W, Höffler D. Dosierung und Elimination von Dextran 40 bei Hämodialysepatienten. Klinische Wochenschrift 53: 523–527, 1975
Köhler H, Zschiedrich H, Linfante A, Appel F, Pitz H, Clasen R. Die Elimination von Hydroxyäthylstärke 200/0.5, Dextran 40 und Oxypolygelatine. Klinische Wochenschrift 60: 293–301, 1982
Korttila K, Groehn P, Gordin A, Sundberg S, Salo H, et al. Effect of hydroxyethyl starch and dextran on plasma volume and blood hemostasis and coagulation. Journal of Clinical Pharmacology 24: 273–282, 1984
Kroemer H, Haass A, Müller K, Jäger H, Wagner EM, et al. Hemodilution therapy in ischemic stroke: plasma levels and plasma viscosity during long term infusion of dextran 40 or hydroxyethylstarch 200/0.5. European Journal of Clinical Pharmacology, submitted, 1987
Lederer K, Huber C, Dunky M, Fink JK, Ferber HP, et al. Studies on hydroxyethylstarch part 1: molecular characterization by size exclusion chromatography coupled with low-angle laser light scattering. Arzneimittel-Forschung 35: 610–614, 1985
Lindblad B, Abisch E, Bergqvist D. The pharmacokinetics of subcutaneous dihydroergotamine with and without a dextran 70 infusion. European Journal of Clinical Pharmacology 24: 813–818, 1983
Lindblad G, Falk J. Konzentrationsverlauf von Hydroxyäthylstärke und Dextran im Serum und Lebergewebe von Kaninchen und die histopathologischen Folgen der Speicherung von Hydroxyäthlstärke. Infusionstherapie 3: 301–310, 1976
Maguire LC, Strauss RG, Koepke JA, Bowman RJ, Zelenski KR, et al. The elimination of hydroxyethyl starch from the blood of donors experiencing single or multiple intermittent-flow centrifugation leukapheresis. Transfusion 21: 347–353, 1981
Metcalf W, Papadopoulos A, Tufaro R, Barth A. A clinical physiologic study of hydroxyethyl starch. Surgery, Gynecology and Obstetrics 131: 255–267, 1970
Mishler JM. Pharmakokinetik mittelmolekularer Hydroxyäthylstärke (HAES 200/0.5). Infusionstherapie 7: 96–102, 1980
Mishler JM. Synthetic plasma volume expanders — their pharmacology, safety and clinical efficacy. Clinics in Haematology 13: 75–92, 1984a
Mishler JM. Pharmacological effects produced by the acute and chronic administration of hydroxyethylstarch. Journal of Clinical Apheresis 2: 52–62, 1984b
Mishler JM, Borberg H, Emerson PM, Gross R. Hydroxyethylstarch: an agent for hypervolemic shock treatment II. Urinary excretion in normal volunteers following three consecutive daily infusions. British Journal of Clinical Pharmacology 4: 591–595, 1977a
Mishler JM, Borberg H, Emerson PM, Gross R. Hydroxyethylstarch: an agent for hypovolemic shock treatment I. Serum concentrations in normal volunteers following three consecutive daily infusions. Journal of Surgical Research 23: 239–245, 1977b
Mishler JM, Parry ES, Petrie A. Plasma clearance and renal excretion of erythrocyte cryoprotectant hydroxyethylated amylopectin. British Journal of Haematology 40: 231–237, 1978
Mishler JM, Parry ES, Sutherland BA, Bushrod JR. A clinical study of low molecular weight hydroxyethylstarch, a new plasma expander. British Journal of Clinical Pharmacology 7: 619–622, 1979a
Mishler JM, Ricketts CR, Parkhouse EJ. Changes in molecular composition of circulating hydroxyethylstarch following consecutive daily infusions in man. British Journal of Clinical Pharmacology 7: 505–509, 1979b
Mishler JM, Ricketts CR, Parkhouse EJ. Post transfusion survival of hydroxyethylstarch 450/0.7 in man: a long term study. Journal of Clinical Pathology 33: 155–159, 1980
Muchmore E, Bonhard K, Kothe N. Distribution and clearance from the body of an oxypolygelatine plasma substitute determined by radioactive tracer study in chimpanzees. Arzneimittel-Forschung 33: 1552–1554, 1983
Ring J, Messmer K. Incidence and severity of anaphylactoid reactions to colloid volume substitute. Lancet 1: 467–469, 1977
Schwarz JA, Koch W, Bühler V, Kaumeier S. Pharmacokinetics of low molecular (monovalent) dextran (Dx1) in volunteers. International Journal of Clinical Pharmacology, Therapy and Toxicology 19: 358–367, 1981
Scott TA, Melvin E. Determination of dextran with anthrone. Analytical Chemistry 25: 1656–1661, 1953
Sum CY, Lai CM, Yacobi A, Kalhorn TF. Chemical characterization of the persistent fraction of hydroxyethyl starch in rat serum and spleen. Life Sciences 33: 1989–1994, 1983b
Sum CY, Mai K, Kam ST, Lai CM, Yacobi A, et al. Gas liquid Chromatographic analysis of hydroxyethyl starch. Journal of Chromatography 254: 187–194, 1983a
Thompson WL. Erwiderung auf die Studie von G. Lindblad und J. Falk: Konzentrationsverlauf von Hydroxyäthylstärke und Dextran in Serum und Lebergewebe von Kaninchen und die histopathologischen Folgen der Speicherung von Hydroxyäthylstärke. Infusionstherapie 3: 102–103, 1977
Thorén L. The dextrans-clinical data. Development of Biological Standardization 48: 157–167, 1981
Wallenius G. Quantitative determination of dextran in blood and urine. Acta Societatis Medicorum Uppsaliensis 59 (Suppl. 4): 5–89, 1954
Yacobi A, Stoll RG, Sun CY, Lay CM, Gupta SD, et al. Pharmacokinetics of hydroxyethyl starch in normal subjects. Journal of Clinical Pharmacology 22: 206–212, 1982
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Klotz, U., Kroemer, H. Clinical Pharmacokinetic Considerations in the Use of Plasma Expanders. Clin-Pharmacokinet 12, 123–135 (1987). https://doi.org/10.2165/00003088-198712020-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-198712020-00003