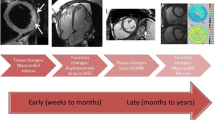
Abstract
Several anticancer drugs have been associated with cardiac toxicity, especially the anthracyclines and trastuzumab. The pathogenesis of anthracycline-associated toxicity has been well described, whereas the mechanism of trastuzumab-associated toxicity is unknown. Although routine cardiac imaging studies (e.g. echocardiogram or multiple gated acquisition scans) may identify subclinical evidence of myocardial dysfunction, available data do not support their routine use for monitoring asymptomatic patients undergoing cancer therapy. Other modalities such as nuclear medicine scintigraphy with indium-111-antimyosin antibody and endomyocardial biopsy have been shown to be useful in identifying early cardiac damage, but their routine use is limited by practical considerations such as feasibility and cost. Consequently, there is significant interest in developing simple and reproducible methods for identifying patients at risk for treatment-induced myocardial damage. Available data suggest that circulating markers such as troponins and natriuretic peptides could potentially be useful for this purpose. Measurement of plasma troponin levels are commonly used in clinical practice in order to provide diagnostic and prognostic information in patients with myocardial ischaemia. Elevated levels may likewise correlate with anthracycline-induced cardiac damage, although plasma levels are only minimally elevated (well below that associated with ischaemia), and elevations may persist for weeks or months after anthracycline exposure. Clinical trials are currently evaluating the role of these markers in predicting both early and late, clinical and subclinical damage associated with anthracyclines and trastuzumab.




Similar content being viewed by others
References
Early Breast Cancer Trialists’ Collaborative Group. Polychemotherapy for early breast cancer: an overview of the randomised trials. Lancet 1998; 352: 930–42
Levine MN, Bramwell VH, Pritchard KI, et al. Randomized trial of intensive cyclophosphamide, epirubicin, and fluorouracil chemotherapy compared with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer. J Clin Oncol 1998; 16: 2651–8
French Epirubicin Study Group. Benefit of a high-dose epirubicin regimen in adjuvant chemotherapy for node-positive breast cancer patients with poor prognostic factors: 5-year follow-up results of French Adjuvant Study Group 05 randomized trial. J Clin Oncol 2001; 19: 602–11
A’Hern RP, Smith IE, Ebbs SR. Chemotherapy and survival in advanced breast cancer: the inclusion of doxorubicin in Cooper type regimens. Br J Cancer 1993; 67: 801–5
Fossati R, Confalonieri C, Torri V, et al. Cytotoxic and hormonal treatment of metastatic breast cancer: a systematic review of published randomized trials involving 31 510 women. J Clin Oncol 1998; 16: 3439–60
Myers C. The role of iron in doxorubicin-induced cardiomyopathy. Semin Oncol 1998; 25Suppl. 10: 10–4
Shan K, Lincoff AM, Young JB. Anthracycline-induced cardiotoxicity. Ann Intern Med 1996; 125: 47–58
Billingham ME, Mason JW, Bristow MR, et al. Anthracycline cardiomyopathy monitored by morphologic changes. Cancer Treat Rep 1978; 62: 865–72
Doroshow JH, Locker GY, Myers CE. Enzymatic defences of the mouse heart against reactive oxygen metabolites: alterations produced by doxorubicin. J Clin Invest 1980; 65: 128–35
Sparano JA. Use of dexrazoxane and other strategies to prevent cardiomyopathy associated with doxorubicin-taxane combinations. Semin Oncol 1998; 25: 66–71
Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med 1979; 91: 710–7
Swain SM. Adult multicenter trials using dexrazoxane to protect against cardiac toxicity. SeminOncol 1998; 25Suppl. 10: 43–7
Swain SM, Shaley FS, Gerber MC, et al. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J Clin Oncol 1997; 10: 117–27
Steinherz LJ, Steinherz PG, Tan CTC, et al. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA 1991; 266: 1672–7
Zambetti M, Moliterni A, Materazzo C, et al. Long-term cardiac sequelae in operable breast cancer patients given adjuvant chemotherapy with or without doxorubicin and breast irradiation. J Clin Oncol 2001; 19: 37–43
Carter P, Presta L, Gorman CM, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci U S A 1992; 89: 4285–9
Hung MC, Lau YK. Basic science of HER-2/neu: a review. Semin Oncol 1999; 26: 51–9
Tzahar E, Waterman H, Chen X, et al. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol 1996; 16: 5276–87
Karunagaran D, Tzahar E, Beerli RR, et al. ErbB-2 is a common auxiliary subunit of NDF and EGF receptors: implications for breast cancer. EMBO J 1996; 15: 254–64
Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987; 235: 177–82
Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344: 783–92
Sparano JA. Cardiac toxicity of trastuzumab (herceptin): implications for the design of adjuvant trials. Semin Oncol 2001; 28Suppl. 3: 20–7
Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol 2002; 20: 1215–21
Moreb JS, Oblon DJ. Outcome of clinical congestive heart failure induced by anthracycline chemotherapy. Cancer 1992; 70: 2637–41
Zhao YY, Sawyer DR, Baliga RR, et al. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ERbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem 1998; 272: 10261–9
Carraway KL. Involvement of the neuregulins and their receptors in cardiac and neural development. Bioessays 1996; 18: 263–6
Erickson SL, O’Shea KS, Ghaboosi N, et al. ErbB3 is required for normal cerebellar and cardiac development: a comparison with ErbB2- and heregulin-deficient mice. Development 1997; 124: 4999–5011
Zhao YY, Feron O, Dessy C, et al. Neuregulin signaling in the heart. Dynamic targeting of erbB4 to caveolar microdomains in cardiac myocytes. Circ Res 1999; 84: 1380–7
McKillop JH, Bristow MR, Goris ML, et al. Sensitivity and specificity of radionuclide ejection fractions in doxorubicin cardiotoxicity. Am Heart J 1983; 106: 1048–56
Erselcan T, Kairemo KJ, Wiklund TA, et al. Subclinical cardiotoxicity following adjuvant dose-escalated FEC, high-dose chemotherapy, or CMF in breast cancer. Br J Cancer 2000; 82: 777–81
Kremer LC, Tiel-van Buul MM, Ubbink MC, et al. Indium-111-antimyosin scintigraphy in the early detection of heart damage after anthracycline therapy in children. J ClinOncol 1999; 17: 1208–11
Mackay B, Ewer MS, Carrasco CH, et al. Assessment of anthracycline cardiomyopathy by endomyocardial biopsy. Ultrastruct Pathol 1994; 18: 203–11
Billingham ME, Mason JW, Bristow MR, et al. Anthracycline cardiomyopathy monitored by morphologic changes. Cancer Treat Rep 1978; 62: 865–72
Pegelow CH, Popper RW, de Wit SA, et al. Endomyocardial biopsy to monitor anthracycline therapy in children. J Clin Oncol 1984; 2: 443–6
Perry SV. The regulation of contractile activity in muscle. Biochem Soc Trans 1979; 7: 593–617
Hamm CW. New serum markers for acute myocardial infarction. N Engl J Med 1994; 331: 607–8
Goldmann BU, Christensen RH, Hamm CW, et al. Implications of troponin testing in clinical medicine. Curr Control Trials Cardiovasc Med 2001; 2: 75–84
Katus HA, Diederich KS, Schwarz F, et al. Influence of reperfusion on serum concentrations of cytosolic creatine kinase and structural myosin light chains in acute myocardial infarction. Am J Cardiol 1987; 60: 440–5
Katus HA, Remppis A, Scheffold T, et al. Intracellular compart-mentation of cardiac troponin T and its release kinetics in patients with reperfused and nonreperfused myocardial infarction. Am J Cardiol 1991; 67: 1360–7
Adams III JE, Schechtman KB, Landt Y, et al. Comparable detection of acute myocardial infarction by creatine kinase MB isoenzyme and cardiac troponin I. Clin Chem 1994; 40: 1291–5
Wu AHB, Lane PL. Meta-analysis in clinical chemistry: validation of troponin T as a marker for ischemic heart disease. Clin Chem 1995; 41: 1228–33
Falk E. Unstable angina with fatal outcome: dynamic coronary thrombosis leading to infarction and/or sudden death: autopsy evidence of recurrent mural thrombosis with peripheral embolization culminating in total vascular occlusion. Circulation 1985; 71: 699–708
Davies MJ, Thomas AC, Knapman PA, et al. Intramyocardial platelet aggregation in patients with unstable angina suffering ischemic cardiac death. Circulation 1986; 73: 418–27
The Joint European Society of Cardiology/American College of Cardiology Committee. Myocardial infarction redefined a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 2000; 36: 959–69
Olatidoye AG, Wu AH, Feng YJ, et al. Prognostic role of troponin T versus troponin I in unstable angina pectoris for cardiac events with meta-analysis comparing published studies. Am J Cardiol 1998; 98: 1853–9
Polanczyk CA, Lee TH, Cook EF, et al. Cardiac troponin I as a predictor of major cardiac events in emergency department patients with acute chest pain. J Am Coll Cardiol 1998; 32: 8–14
Greenson N, Macoviak J, Krishnaswamy P, et al. Usefulness of cardiac troponin I inpatients undergoing open heart surgery. Am Heart J 2001; 141: 447–55
Saadeddin SM, Habbab MA, Sobki SH, et al. Minor myocardial injury after elective uncomplicated successful PTCA with or without stenting: detection by cardiac troponins. Catheter Cardiovasc Interv 2001; 53: 188–92
Schluter T, Baum H, Plewan A, et al. Effects of implantable cardioverter defibrillator implantation and shock application on biochemical markers of myocardial damage. Clin Chem 2001; 47: 459–63
Manolis AS, Vassilikos V, Maounis T, et al. Detection of myocardial injury during radiofrequency catheter ablation by measuring cardiac troponin I levels: procedural correlates. J Am Coll Cardiol 1999; 34: 1099–105
Salim A, Velmahos GC, Jindal A, et al. Clinically significant blunt cardiac trauma: role of serum troponin levels combined with electrocardiographic findings. J Trauma 2001; 50: 237–43
Del Carlo CH, O’Connor CM. Cardiac troponins in congestive heart failure. Am Heart J 1999; 138: 646–53
Meyer T, Binder L, Hruska N, et al. Cardiac troponin I elevation in acute pulmonary embolism is associated with right ventricular dysfunction. J Am Coll Cardiol 2000; 36: 1632–6
Lauer B, Niederau C, Kuhl U, et al. Cardiac troponin T in patients with clinically suspected myocarditis. J Am Coll Cardiol 1997; 30: 1354–9
Dengler TJ, Zimmermann R, Braun K, et al. Elevated serum concentrations of cardiac troponin in acute allograft rejection after human heart transplantation. J Am Coll Cardiol 1998; 32: 405–12
Ver Elst KM, Spapen HD, Nguyen DN, et al. Cardiac troponins I and T are biological markers of left ventricular dysfunction in septic shock. Clin Chem 2000; 46: 650–7
Venugopal J. Cardiac natriuretic peptides - hope or hype? J Clin Pharm Ther 2001; 26: 15–31
Hama N, Itoh H, Shirakami G, et al. Rapid ventricular induction of brain natriuretic peptide gene expression in experimental acute myocardial infarction. Circulation 1995; 92: 1558–64
Fleming SM, O’Gorman T, O’Byrne L, et al. Cardiac troponin I and N-terminal pro-brain natriuretic peptide in relation to fetal heart rate abnormalities during labor. Pediatr Cardiol 2001; 22: 393–6
Hammerer-Lercher A, Neubauer E, Muller S, et al. Head-to-head comparison of N-terminal pro-brain natriuretic peptide, brain natriuretic peptide and N-terminal pro-atrial natriuretic peptide in diagnosing left ventricular dysfunction. Clin Chim Acta 2001; 310: 193–7
Hammerer-Lercher A, Puschendorf B, Mair J. Cardiac natriuretic peptides: new laboratory parameters in heart failure patients. Clin Lab 2001; 47: 265–77
Darbar D, Davidson NC, Gillespie N, et al. Diagnostic value of B-type natriuretic peptide concentrations in patients with acute myocardial infarction. Am J Cardiol 1996; 78: 284–7
McDonagh TA, Cunningham AD, Morrison CE, et al. Left ventricular dysfunction, natriuretic peptides, and mortality in an urban population. Heart 2001; 86: 21–6
Fleischer D, Espiner EA, Yandle TG, et al. Rapid assay of plasma brain natriuretic peptide in the assessment of acute dyspnoea. N Z Med J 1997; 110: 71–4
Cowie MR, Struthers AD, Wood DA, et al. Value of natriuretic peptides in assessment of patients with possible new heart failure in primary care. Lancet 1997; 350: 1349–53
Richards AM, Doughty R, Nicholls MG, et al. Neurohumoral prediction of benefit from carvedilol in ischaemic left ventricular dysfunction. Australia-New Zealand Heart Failure Group. Circulation 1999; 99: 786–92
Richards AM, Doughty R, Nicholls MG, et al. Plasma N-terminal pro-brain natriuretic peptide and adrenomedullin: prognostic utility and prediction of benefit from carvedilol in chronic ischaemic left ventricular dysfunction. Australia- New Zealand Heart Failure Group. J Am Coll Cardiol 2001; 37: 1781–7
Stanek B, Frey B, Hulsmann M, et al. Prognostic evaluation of neurohumoral plasma levels before and during beta-blocker therapy in advanced left ventricular dysfunction. J Am Coll Cardiol 2001; 38: 436–42
Herman EH, Lipshultz SE, Rifai N, et al. Use of cardiac troponin T levels as an indicator of doxorubicin-induced cardiotoxicity. Cancer Res 1998; 58: 195–7
Fink FM, Genser N, Fink C, et al. Cardiac troponin T and creatine kinase MB mass concentrations in children receiving anthracycline chemotherapy. Med Pediatr Oncol 1995; 25: 185–9
Lipshultz SE, Rifai N, Sallan SE, et al. Predictive value of cardiac troponin T in pediatric patients at risk for myocardial injury. Circulation 1997; 96: 2641–8
Lipshultz S, Sallan S, Dalton V, et al. Elevated serum cardiac troponin-T as a marker for active cardiac injury during therapy for childhood acute lymphoblastic leukemia (ALL) [abstract no. 2191]. Proc Am Soc Clin Oncol 1999; 18: 568a
Hughes-Davies L, Sacks D, Rescigno J, et al. Serum cardiac troponin T levels during treatment of early-stage breast cancer. J Clin Oncol 1995; 13: 2582–4
Auner HW, Tinchon C, Sill H, et al. Serum troponin T as an indicator of anthracycline cardiotoxicity in adults [abstract no. 1552]. Proc Am Soc Clin Oncol 2001; 20: 389a
Nag SM, Briscoe K, Desouza P. A pilot study of troponin T as a prognostic marker in patients treated with 5-flurouracil [abstract no. 3126]. Proc Am Soc Clin Oncol 2001; 20: 344b
Hirsch R, Landt Y, Porter S, et al. Cardiac troponin I in pediatrics: normal values and potential use in the assessment of cardiac injury. J Pediatr 1997; 130: 872–87
Mathew P, Suarez W, Kip K, et al. Is there a potential role for serum cardiac troponin I as a marker for myocardial dysfunction in pediatric patients receiving anthracycline-based therapy? A pilot study. Cancer Invest 2001; 19: 352–9
Sedky LM, Hamada E, Selim H, et al. The value of troponin-I measurement in assessment of anthracycline induced cardiotoxicity among breast cancer patients [abstract 1772]. Proc Am Soc Clin Oncol 2001; 20: 6b
Cardinale D, Sandri MT, Martinoni A, et al. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J Am Coll Cardiol 2000; 36: 517–22
Benvenuto GM, La Vecchia L, Morandi P, et al. Assessment of cardiotoxicity of high dose cyclophosphamide with electrocardiographic, echocardiographic, and troponin I monitoring in patients with breast tumors. Ital Heart J 2000; 1: 1457–63
Nousiainen T, Jantunen E, Vanninen E, et al. Acute neurohumoral and cardiovascular effects of idarubicin in leukemia patients. Eur J Haematol 1998; 61: 347–53
Nousiainen T, Jantunen E, Vanninen E, et al. Natriuretic peptides as markers of cardiotoxicity during doxorubicin treatment for non-Hodgkin’s lymphoma. Eur J Haematol 1999; 62: 135–41
Suzuki T, Hayashi D, Yamazaki T, et al. Elevated B-type natriuretic peptide levels after anthracycline administration. Am Heart J 1998; 136: 362–3
Okumura H, Iuchi K, Yoshida T, et al. Brain natriuretic peptide is a predictor of anthracycline-induced cardiotoxicity. Acta Haematol 2000; 104: 158–63
Hayakawa H, Komada Y, Hirayama M, et al. Plasma levels of natriuretic peptides in relation to doxorubicin-induced cardiotoxicity and cardiac function in children with cancer. Med Pediatr Oncol 2001; 37: 4–9
Berry G, Billingham M, Alderman E, et al. The use of cardiac biopsy to demonstrate reduced cardiotoxicity in AIDS Kaposi’s sarcoma patients treated with pegylated liposomal doxorubicin. Ann Oncol 1998; 9: 711–6
Acknowledgements
The authors have received no funding for the preparation of this manuscript, nor have any conflicts of interest directly relevant to the contents of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sparano, J.A., Brown, D.L. & Wolff, A.C. Predicting Cancer Therapy-Induced Cardiotoxicity. Drug-Safety 25, 301–311 (2002). https://doi.org/10.2165/00002018-200225050-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200225050-00001