
Upregulation of programmed death ligand 1 and epidermal growth factor receptor is associated with poor prognosis in gastric cancer
Introduction
Gastric cancer is one of the leading causes of cancer-related death worldwide (1). The incidence rate of gastric carcinoma is particularly high in Asian areas, where specific environmental and genetic triggers differ gastric cancer patients from those in Western countries (2). With the widespread application of endoscopic technology, gastric cancer patients are earlier diagnosed than before. Whereas, overall survival has still not been much prolonged and major breakthroughs have yet to be made since the invention of trastuzumab targeting human epidermal growth factor receptor 2 (HER2) for only a small portion of patients with HER2 overexpression (3).
Immune escape plays a pivotal role during tumor progression. Costimulatory molecules, otherwise known as immune checkpoints that normally maintain self-tolerance and limit collateral inflammatory damage, are reported to be co-opted by cancer cells to evade immune annihilation via inducing T cell apoptosis (4). Programmed death ligand-1 (PD-L1), located on the surface of cancer cells, can bind with programmed death 1 (PD-1) on the surface of various immune cells and consequently activate a typical PD-1/PD-L1 immune checkpoint pathway, establishing inhibitory effects on anti-tumor immune activity (5). Expectations for tumor immunotherapy were dramatically raised upon the emergence of checkpoint blockade antibodies targeting the PD-1/PD-L1 pathway (6). PD-1/PD-L1 inhibitors were first used in advanced melanoma patients and the inspiring results fueled numerous clinical trials (7-12). It was suggested that PD-L1 expression on tumor cells is related to patient objective response (13). By far, the American Food and Disease Administration (FDA) has officially approved five PD-1/PD-L1 antibodies, namely Opdivo (nivolumab), Keytruda (pembrolizumab), Tecentriq (atezolizumab), Bavencio (avelumab) and Imfinzi (durvalumab). Indications covered malignant melanoma, non-small cell lung cancer, head and neck squamous cell carcinoma, classical Hodgkin’s lymphoma, renal cell carcinoma, uroepithelium carcinoma and Merkel cell carcinoma. Recently, Keytruda was granted an accelerated approval by the FDA for both adult and pediatric patients who have unresectable or metastatic, microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) solid tumors. It is considered a broad-spectrum anticancer drug based on biomarkers regardless of the tumor’s original location. It provides new hope for gastric cancer patients seeking help from other than conventional chemotherapy, especially for HER2 negative patients who cannot benefit from trastuzumab. Plus, it was reported that MSI indicated high PD-L1 expression in gastric cancer patients (14). Additional approvals are expected to broaden the clinical scope of PD-1/PD-L1 blockade and benefit more patients. A number of studies involving Asian gastric cancer patients arrived at the same conclusion that PD-L1 upregulation indicates poor prognosis (15-21), while others reported opposite results, especially regarding Caucasian patients (22,23). The clinicopathologic influence of PD-L1 in gastric cancer has not been fully elucidated and the explicit mechanism underlying how PD-L1 affects prognosis still waits to be unveiled.
The human epidermal growth factor receptor (EGFR), also known as HER-1, is a cell membrane tyrosine kinase receptor and a member of the HER family that is involved during the tumorigenesis and progression of multiple types of human cancer (24). Upregulation of EGFR in human cancer can lead to uncontrolled cell growth and division (25). It is widely acknowledged that EGFR-targeting tyrosine kinase inhibitors (TKIs) benefited numerous patients, especially in lung adenocarcinoma patients with EGFR mutations, for whom EGFR inhibiting TKI is now recommended for first-line treatment. However, similar achievements were not obtained in gastric cancer patients. Providing that a strong correlation between EGFR protein expression and gene copy number was proved in gastric cancer (26,27), and that EGFR amplification had an adverse prognostic impact (28), EGFR-targeting is theoretically feasible and effective for gastric patients with EGFR protein overexpression or gene amplification. Yet it was pitifully not the case under clinical circumstances. In gastric cancer patients, monoclonal antibodies cetuximab, panitumumab and matuzumab targeting EGFR, and the dual EGFR and HER2 tyrosine kinase inhibitor lapatinib, did not yield rather satisfactory outcomes. The prognostic significance of EGFR in gastric cancer patients remains controversial. It has been reported that EGFR overexpression is associated with worse prognosis (26,29-36), while some claimed otherwise with contradictory results (27,37). Others concluded that EGFR did not have a significant prognostic impact (38-43).
As is mentioned above, inconsistent results were obtained by a number of studies concerning the prognostic significance of PD-L1 and EGFR. In the current study, we examined the association of PD-L1 and EGFR with clinicopathologic characteristics as well as overall survival in Chinese gastric cancer patients. We also attempted to determine whether there existed a certain relationship between PD-L1 and EGFR expression.
Methods
Patient samples
Commercially available gastric cancer tissue microarrays were purchased (HStm-Ade180Sur-03, Shanghai Outdo Biotech. Co., Ltd., Shanghai, China) with patient profiles, available at: http://www.outdobiotech.com/. Informed consent was gained before sample collection according the company website. Specimens were acquired from 90 gastric cancer patients who received surgical treatment from July 2006 to April 2007. Each case provided two pairing spots of tumor tissue and corresponding adjacent normal tissue. Profiles provided overall survival, gender, age, pathological differentiation, tumor size, tumor location, Borrmann classification, vascular invasion, lymph node invasion, distant metastasis (before surgery), TNM stages and clinical stage according to the 7th AJCC standard. Overall survival (OS) was calculated from the date of surgery till death or the end of follow-up period. Patient anonymity was strictly preserved. The outcome of our study did not and will not affect the future management of the patients. The study was conducted in accordance with the Helsinki Declaration as revised in 2013, available at: http://www.wma.net/en/30publications/10policies/b3/%20index.html.
Immunohistochemistry
The primary antibodies used for immunohistochemistry were commercially available EGFR antibody (RMA-0554, Fuzhou Maixin Biotech. Co., Ltd, Fuzhou, China) and PD-L1 antibody (NBP1-76769, Novus Biologicals LLC, CO, USA). Formalin-fixed, paraffin-embedded microarray slides were heated at 85 °C for 1 h and then cooled for 20 min at room temperature. The slides were immersed in dimethylbenzene three times for deparaffinage for 15 min each and then hydrated in 100%, 95% and 75% ethanol consecutively for 5 min. For antigen retrieval, slides were heated at 125 °C for 5 min in 2% EDTA-citrate antigen retrieval solution (MVS-0099, Fuzhou Maixin Biotech. Co., Ltd., Fuzhou, China) in a pressure cooker. After being rinsed by PBS (PBS-0061, Fuzhou Maixin Biotech. Co., Ltd., Fuzhou, China), the slides were immersed in hydrogen peroxide at room temperature for 30 min for endogenous peroxidase ablation, followed by incubation with 3% BSA at 37 °C for 30 min to block nonspecific binding. Then they were incubated with primary antigens at 4 °C for 14 h. PD-L1 antibody was diluted to 1:200 using antibody diluent (ABD-0030, Fuzhou Maixin Biotech. Co., Ltd., Fuzhou, China) and EGFR antibody was applied without concentration adjustment. A MaxVisionTM rapid immunohistochemistry kit (KIT-5020, Fuzhou Maixin Biotech. Co., Ltd., Fuzhou, China) was applied and the secondary antibody binding process was conducted according to the manufacturer’s protocols. A DAB substrate kit (DAB-0031, Fuzhou Maixin Biotech. Co., Ltd., Fuzhou, China) was applied and the staining process was conducted according to the manufacture’s protocols.
Scoring system of immunostaining
Immunostaining was evaluated by two professional pathologists independently who had no access to patient clinical files, and discrepancies were solved by joint review. For both PD-L1 and EGFR, only membranous staining of tumor cells was considered positive. We adapted the conventional Histoscore (H-score) calculation. It was determined by a semi-quantitative assessment of both the intensity of staining (graded as: 0, non-staining; 1, weak; 2, median; or 3, strong using adjacent normal mucosa as the median) and the percentage of positive cells. The range of possible scores was from 0 to 300. Expression level of each component was categorized as low or high according to the cutoff value of the H-score.
Statistical analysis
The statistical analysis was performed using SPSS 21 software (SPSS Inc., Chicago, IL, USA). Comparisons of mean protein expression levels between tumor tissue and adjacent normal tissue were done by rank sum test. Comparisons of protein expression in patients with different clinicopathologic characteristics were done by Chi-square test and Fisher’s exact test. The survival curves for OS were derived from Kaplan-Meier estimates and compared by log-rank tests. Cox proportional hazard regression model was applied to explore the prognostic effect of protein expression as well as other clinicopathologic characteristics. All comparisons were done on both sides and P values <0.05 were deemed statistically significant. Odds ratios (OR) and 95% confidence intervals (CI) were calculated.
Results
PD-L1 and EGFR expression are both upregulated in gastric cancer
PD-L1 expression was examined in 180 samples of tumor tissues and corresponding adjacent normal tissues. Expression levels were evaluated by the scoring system mentioned above. Among 90 gastric cancer samples, 4 of them lacked enough tumor tissue to be examined, leaving 86 valid samples. Typical samples of immunostaining are exhibited (Figure 1). Rank sum test showed PD-L1 expression levels in gastric cancer tissues were significantly higher than those in normal tissues (P=0.036).

EGFR expression was examined in a second slide from the same batch. Immunostaining were evaluated through the same method (Figure 1). In this slide, 6 of 90 tumor samples lacked enough tumor tissue to be examined, leaving 84 valid samples. Rank sum test yielded similar results. EGFR expression levels in gastric cancer tissues were significantly higher than those in normal tissues (P<0.001).
Relationships of PD-L1 and EGFR expression with clinicopathologic characteristics in gastric cancer
In the current study, we applied an evaluation system using H-score to semi-quantify protein expression levels. Samples stained with PD-L1 antibody were deemed PD-L1 positive when H-score reached 15, and samples stained with EGFR antibody were deemed EGFR overexpression when H-score reached 215. Cutoff values were determined using Cutoff Finder (44). Cases lacking complete data were removed during statistical analysis. Chi-square test was conducted to explore PD-L1 and EGFR expression in gastric cancer patients with different clinicopathologic characteristics (Table 1).
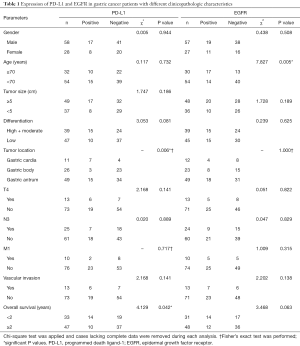
Full table
We detected 29.1% (29/86) PD-L1 positivity and 35.7% (30/84) EGFR overexpression rate in gastric cancer patients. Chi-square test and Fisher’s exact test revealed that PD-L1 positivity was potentially related with tumor location (P=0.006) and shorter survival (χ2=4.129, P=0.042). EGFR overexpression was found to be potentially related with older age (χ2=7.827, P=0.005). EGFR overexpression also tended to lead to shorter survival (χ2=3.468, P=0.063). No significant relationship was found between PD-L1 or EGFR expression with other clinicopathologic characteristics.
Impact of PD-L1 and EGFR expression on overall survival in gastric cancer patients
The follow-up period in the current study is 98 months. The median follow-up time is 43 months and the median survival time is 43.0 months. In order to determine the prognostic impact of PD-L1 and EGFR overexpression along with other clinicopathologic characteristics on gastric cancer patients, we performed Kaplan-Meier analysis, univariate Cox regression and multivariate Cox regression to calculate the hazard ratios (HRs) of several parameters.
Kaplan-Meier estimates and log-rank tests revealed that patients with PD-L1 positive expression had significantly shorter median survival time (Figure 2; 65.0 vs. 21.0 months, log-rank χ2=4.074, P=0.044). Patients with EGFR overexpression presented similar results (Figure 2, 69.0 vs. 20.0 months, log-rank χ2=7.668, P=0.006).

In univariate Cox regression models (Table 2), PD-L1 positive expression (HR =1.793, P=0.049), EGFR overexpression (HR =2.178, P=0.008), low differentiation (HR =2.019, P=0.016), depth of tumor invasion or later T stage (HR =2.028, P=0.038), lymph node invasion or later N stage (HR =2.653, P=0.001), distant metastasis (HR =2.799, P=0.006) and vascular invasion (HR =3.224, P<0.001) were associated with shorter overall survival. Larger tumor size tended to suggest shorter overall survival (HR =1.688, P=0.063).
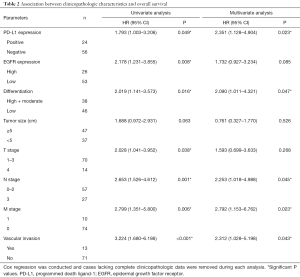
Full table
Multivariate Cox regression demonstrated that PD-L1 overexpression (HR =2.351, P=0.023), low differentiation (HR =2.090, P=0.047), lymph node invasion or later N stage (HR =2.253, P=0.045), distant metastasis (HR =2.792, P=0.023) and vascular invasion (HR =2.312, P=0.043) were potential independent factors for worse prognosis (Table 2).
No association was found between PD-L1 and EGFR expression
Spearman’s rank correlation was performed to determine whether there existed a certain relationship between PD-L1 and EGFR expression. The result was negative with a P value almost equaling 1. However, it is worth mentioning that 8 patients with coexpression of both PD-L1 and EGFR tended to have shorter overall survival than the rest (Figure 2, 12 vs. 43 months, log-rank χ2=3.468, P=0.063).
Discussion
In the current study, it was demonstrated that both PD-L1 and EGFR were upregulated in gastric cancer. Expression of PD-L1 was correlated with tumor location and EGFR with patient age. Cox regression revealed that PD-L1 expression, EGFR expression, differentiation, depth of tumor invasion, lymph node invasion, distant metastasis and vascular invasion had significant impacts on patient overall survival, whereas PD-L1 expression, lymph node invasion, distant metastasis and vascular invasion were potential independent factors for worse prognosis.
The identification of PD-1/PD-L1 pathway shed new light into cancer immunotherapy and last decade witnessed the revolutionary success of PD-1/PD-L1 antibodies in cancer patients. Previous studies concerning PD-L1 expression in gastric cancer patients provided detailed information about its correlation with clinicopathologic parameters and prognostic significance. In accordance with our findings, several studies found increased PD-L1 expression in gastric cancer tissue compared with normal tissue (15,16,18,23). Elevated expression of PD-L1 was identified as an adverse prognostic factor in most cases (15-21), except for a study from Korea showing opposite results and another study focusing on Caucasian patients (22,23). We also discovered a potential relationship between PD-L1 and tumor location, while others noted certain correlations between PD-L1 expression and Lauren’s classification (45), lymph node invasion (17), depth of invasion (15), clinical stage (16) and tumor size (19). In our study, PD-L1 expression, differentiation, depth of invasion, lymph node invasion, distant metastasis and vascular invasion were revealed to be related with overall survival, which was supported by previous studies (16-19,21). While the question of how PD-L1 affected overall survival was left unanswered over the years, logical assumptions have been proposed by many scholars. It was stated that PD-L1 could promote naïve T cells to develop into activated negative-modulating Tregs, thus hindering antitumor immunity (15,17). Some claimed that PD-L1 could decrease tumor immunogenicity to impede tumor specific T cell response (16,18). Based on the observation that tumor infiltrating lymphocytes (TILs) with elevated PD-1 expression was found in PD-L1 positive tumor tissue, some suggested that PD-L1 could upregulate PD-1 expression on TILs to promote the activation of PD-1/PD-L1 pathway (20,21).
The Cancer Genome Atlas Network data classified gastric cancer into four major types: Epstein-Barr virus positive (EBV+), MSI, genomically stable and chromosome instability. Elevated mutation rates of tumor suppressor genes and hypermethylation of MLH1, which normally functions as a mismatch repair (MMR) gene, were frequently observed in MSI gastric cancer patients (46). Consequently, patients with MSI-H or dMMR solid tumors are suitable candidates for PD-1 blockade treatment as we mentioned earlier. It was estimated that approximately 9% of gastric cancer patients are infected by EBV, defined as EBV-encoded small RNA (EBER) positive gastric cancer or EBV-associated gastric cancer (EBVaGC) (47). EBVaGC presented unique genetic alterations that translated into specific clinicopathological features, including predominance among males, a proximal location in the stomach, lymphoepithelioma-like histology and a favorable prognosis, especially in the Asian population (48-50). EBV-positive gastric cancer showed more CpG methylation and tended to harbor more mutated PIK3CA and ARID1A as well as an amplified 9p24.1 locus, which upregulated JAK2, PD-L1 and PD-L2 (51). A previous study confirmed overexpression and gene amplification of PD-L1 in EBVaGC, suggesting EBV infection could predict PD-1 blockade treatment response (52), which was supported by another study demonstrating that EBV+ or MSI gastric cancer showed significantly higher rates of PD-L1 expression (53). Researchers also found that intratumoral PD-L1 expression was associated with worse survival in EBVaGC patients (54). These findings lead to the inspiring question: are EBVaGC patients also potential candidates for PD-1 blockade treatment?
Much earlier identified and studied than PD-L1, EGFR is revealed to be a transmembrane receptor tyrosine kinase consisting of three domains: an extracellular ligand-binding domain, a lipophilic transmembrane segment, and a cytoplasmic tyrosine kinase domain (55). EGFR overexpression as well as gene amplification was seen in a wide range of carcinomas. It is well established that EGFR is involved in tumorigenesis and progression (56). Scientist realized that EGFR signaling could drive cancer cell growth 40 years ago, and clinical use of EGFR inhibitors flourished ever since. In the current study, we observed that EGFR was overexpressed in gastric cancer tissue compared with normal tissue (34,57). We noticed that older age might be a risk factor for high EGFR expression in gastric cancer patients, in line with a previous study (26). Other EGFR related clinicopathologic features included tumor stage (33), depth of invasion (29,43), tumor location (57), differentiation (26), lymph node invasion (33), distant metastasis (31) and disease recurrence (34). We identified EGFR expression to be associated with worse prognosis for gastric cancer patients, solidifying the findings of a few studies (26,29-32,34). In univariate analysis, EGFR expression, depth of invasion, lymph node invasion, distant metastasis and vascular invasion were potential prognostic factors, as is supported by previous studies (33,34). EGFR exerts its adverse impact on gastric cancer through multiple manners. Some claimed that activation of EGFR by Helicobacter pylori could result in survival of gastric epithelial cells with DNA damage (35), which was supported by another study that inhibiting EGFR led to downregulated Helicobacter-pylori-induced epithelial carcinogenesis (36). Similar results were gained when inhibiting EGFR suppressed its effect on promoting gastric cancer cell survival (31). It was also discovered that EGFR in exosomes secreted from gastric cancer cells could be delivered and integrated on the membrane of liver stromal cells, activating hepatocyte growth factor and facilitating metastasis (58).
Multiple studies demonstrated that PD-L1 protein expression is positively correlated with EGFR gene mutation in lung cancer (59,60). However, we did not find any published work describing the same phenomenon in gastric cancer patients. Gastric cancer is a highly heterogeneous disease. To our knowledge, this is the first study to address the relationship of PD-L1 and EGFR expression in gastric cancer. No correlation or even a slight tendency was discovered between PD-L1 expression and EGFR expression, implying a different mechanism from that in lung cancer. This could be explained that EGFR mutation is detected in a wide range of lung cancer patients who generally respond well to EGFR-TKIs, while EGFR amplification is more often observed in gastric cancer patients who are unable to benefit markedly from EGFR-TKIs. Still, we should not jump to the conclusion that PD-L1 and EGFR pathways have absolutely no interaction with each other in gastric cancer patients. In fact, it was already proposed that PD-L1 expression is partially regulated by EGFR/HER2 pathway in gastric cancer (61). Further cellular and genetic experiments are required for a deeper look into this matter.
There are several limitations in our study. First and foremost, the patient medical files do not include patients’ comorbidity, specific surgical procedures, the extent of lymphadenectomy or surgical complications. As is shown in Table 1, 10 of 90 patients presented distant metastasis before surgery. It is out of question that the ninety patients did not all receive radical surgery. We contacted Shanghai Outdo Biotech. Co., Ltd. to try to improve the medical profiles, but the reply was that the company could neither provide further information nor chase down the original surgical records due to patient anonymity protection. Moreover, the medical files do not include other potential treatment following surgery like chemotherapy and radiation. Last but not least, the files do not include EBV infection status, which would have added much more value to this study. The missing information rendered our results less reliable to some extent. Yet we still believe our survival analysis provides substantial clinical significance in predicting patient prognosis.
Conclusions
We may safely reach the conclusion that both elevated PD-L1 and EGFR protein expression levels in gastric cancer are indicative factors for worse prognosis. PD-L1 might serve as an independent predictive factor in gastric cancer patients. While PD-L1 and EGFR status are reported to be correlated in lung cancer, we did not find any significant correlation between the expression of PD-L1 and EGFR in gastric cancer, indicating that the established interaction mechanisms in lung cancer cannot be simply transferred onto gastric cancer.
Acknowledgments
Funding: This research project was supported by grants from the National Key Technology R&D Program (grant number 2015BAI12B12); the National Natural Science Foundation of China (grant number 31570877 and 31570908); National Natural Science Foundation and Hong Kong and Macao Scholars Cooperative Research Foundation (grant number 31729001); and Jiangsu Engineering Research Center for Tumor Immunotherapy (grant number BM2014404).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.03.15). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Specimens used in this study were commercially available tissue microarrays purchased from Shanghai Outdo Biotech. Co., Ltd. Anonymous patient files could be found at: http://www.outdobiotech.com/. Informed consent was gained before sample collection according the company. Thus there exists no ethical conflict.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Moore MA, Eser S, Igisinov N, et al. Cancer epidemiology and control in North-Western and Central Asia - past, present and future. Asian Pac J Cancer Prev 2010;11:17-32. [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Topalian Suzanne L, Drake Charles G, Pardoll Drew M. Immune Checkpoint Blockade: A Common Denominator Approach to Cancer Therapy. Cancer Cell 2015;27:450-61. [Crossref] [PubMed]
- Sharma P, Allison James P. Immune Checkpoint Targeting in Cancer Therapy: Toward Combination Strategies with Curative Potential. Cell 2015;161:205-14. [Crossref] [PubMed]
- Callahan Margaret K, Postow Michael A, Wolchok Jedd D, Targeting T. Cell Co-receptors for Cancer Therapy. Immunity 2016;44:1069-78. [Crossref] [PubMed]
- Gulley JL, Rajan A, Spigel DR, et al. Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol 2017;18:599-610. [Crossref] [PubMed]
- Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non–Small-Cell Lung Cancer. N Engl J Med 2017;376:2415-26. [Crossref] [PubMed]
- Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017;18:312-22. [Crossref] [PubMed]
- Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med 2017;376:1015-26. [Crossref] [PubMed]
- Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909-20. [Crossref] [PubMed]
- Antonia S, Goldberg SB, Balmanoukian A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol 2016;17:299-308. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- . Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [Crossref] [PubMed]
- Qing Y, Li Q, Ren T, et al. Upregulation of PD-L1 and APE1 is associated with tumorigenesis and poor prognosis of gastric cancer. Drug Des Devel Ther 2015;9:901-9. [Crossref] [PubMed]
- Geng Y, Wang H, Lu C, et al. Expression of costimulatory molecules B7-H1, B7-H4 and Foxp3+ Tregs in gastric cancer and its clinical significance. Int J Clin Oncol 2015;20:273-81. [Crossref] [PubMed]
- Hou J, Yu Z, Xiang R, et al. Correlation between infiltration of FOXP3+ regulatory T cells and expression of B7-H1 in the tumor tissues of gastric cancer. Exp Mol Pathol 2014;96:284-91. [Crossref] [PubMed]
- Wu C, Zhu Y, Jiang J, et al. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem 2006;108:19-24. [Crossref] [PubMed]
- Zhang L, Qiu M, Jin Y, et al. Programmed cell death ligand 1 (PD-L1) expression on gastric cancer and its relationship with clinicopathologic factors. Int J Clin Exp Pathol 2015;8:11084-91. [PubMed]
- Eto S, Yoshikawa K, Nishi M, et al. Programmed cell death protein 1 expression is an independent prognostic factor in gastric cancer after curative resection. Gastric Cancer 2016;19:466-71. [Crossref] [PubMed]
- Tamura T, Ohira M, Tanaka H, et al. Programmed Death-1 Ligand-1 (PDL1) Expression Is Associated with the Prognosis of Patients with Stage II/III Gastric Cancer. Anticancer Res 2015;35:5369-76. [PubMed]
- Kim JW, Nam KH, Ahn SH, et al. Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer 2016;19:42-52. [Crossref] [PubMed]
- Boger C, Behrens HM, Mathiak M, et al. PD-L1 is an independent prognostic predictor in gastric cancer of Western patients. Oncotarget 2016;7:24269-83. [Crossref] [PubMed]
- Carlsson J, Wester K, De La Torre M, et al. EGFR-expression in primary urinary bladder cancer and corresponding metastases and the relation to HER2-expression. On the possibility to target these receptors with radionuclides. Radiol Oncol 2015;49:50-8. [Crossref] [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Kim MA, Lee HS, Lee HE, et al. EGFR in gastric carcinomas: prognostic significance of protein overexpression and high gene copy number. Histopathology 2008;52:738-46. [Crossref] [PubMed]
- Park JS, Kim HS, Bae YS, et al. Prognostic significance and frequency of EGFR expression and amplification in surgically resected advanced gastric cancer. Jpn J Clin Oncol 2016;46:507-16. [Crossref] [PubMed]
- Kandel C, Leclair F, Bou-Hanna C, et al. Association of HER1 amplification with poor prognosis in well differentiated gastric carcinomas. J Clin Pathol 2014;67:307-12. [Crossref] [PubMed]
- Tang D, Liu CY, Shen D, et al. Assessment and prognostic analysis of EGFR, HER2, and HER3 protein expression in surgically resected gastric adenocarcinomas. Onco Targets Ther 2014;8:7-14. [PubMed]
- Nagatsuma AK, Aizawa M, Kuwata T, et al. Expression profiles of HER2, EGFR, MET and FGFR2 in a large cohort of patients with gastric adenocarcinoma. Gastric Cancer 2015;18:227-38. [Crossref] [PubMed]
- Wang D, Wang B, Wang R, et al. High expression of EGFR predicts poor survival in patients with resected T3 stage gastric adenocarcinoma and promotes cancer cell survival. Oncol Lett 2017;13:3003-13. [Crossref] [PubMed]
- Terashima M, Kitada K, Ochiai A, et al. Impact of Expression of Human Epidermal Growth Factor Receptors EGFR and ERBB2 on Survival in Stage II/III Gastric Cancer. Clin Cancer Res 2012;18:5992-6000. [Crossref] [PubMed]
- Al-Moundhri MS, Al-Hadabi I, Al-Mawaly K, et al. Prognostic significance of cyclooxygenase-2, epidermal growth factor receptor 1, and microvascular density in gastric cancer. Med Oncol 2012;29:1739-47. [Crossref] [PubMed]
- Galizia G, Lieto E, Orditura M, et al. Epidermal Growth Factor Receptor (EGFR) Expression is Associated With a Worse Prognosis in Gastric Cancer Patients Undergoing Curative Surgery. World J Surg 2007;31:1458-68. [Crossref] [PubMed]
- Chaturvedi R, Asim M, Piazuelo MB, et al. Activation of EGFR and ERBB2 by Helicobacter pylori Results in Survival of Gastric Epithelial Cells With DNA Damage. Gastroenterology 2014;146:1739-51.e14. [Crossref] [PubMed]
- Sierra JC, Asim M, Verriere TG, et al. Epidermal growth factor receptor inhibition downregulates Helicobacter pylori-induced epithelial inflammatory responses, DNA damage and gastric carcinogenesis. Gut 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Kim JS, Kim MA, Kim TM, et al. Biomarker analysis in stage III–IV (M0) gastric cancer patients who received curative surgery followed by adjuvant 5-fluorouracil and cisplatin chemotherapy: epidermal growth factor receptor (EGFR) associated with favourable survival. Br J Cancer 2009;100:732-8. [Crossref] [PubMed]
- Byeon SJ, Lee HS, Kim M-A, et al. Expression of the ERBB Family of Ligands and Receptors in Gastric Cancer. Pathobiology 2017;84:210-7. [Crossref] [PubMed]
- Fuse N, Kuboki Y, Kuwata T, et al. Prognostic impact of HER2, EGFR, and c-MET status on overall survival of advanced gastric cancer patients. Gastric Cancer 2016;19:183-91. [Crossref] [PubMed]
- Kurokawa Y, Matsuura N, Kawabata R, et al. Prognostic Impact of Major Receptor Tyrosine Kinase Expression in Gastric Cancer. Ann Surg Oncol 2014;21:S584-90. [Crossref] [PubMed]
- Atmaca A, Werner D, Pauligk C, et al. The prognostic impact of epidermal growth factor receptor in patients with metastatic gastric cancer. BMC Cancer 2012;12:524. [Crossref] [PubMed]
- Paliga A, Marginean H, Tessier-Cloutier B, et al. The Prognostic Significance of c-MET and EGFR Overexpression in Resected Gastric Adenocarcinomas. Am J Clin Oncol 2017;40:543-51. [Crossref] [PubMed]
- Czyzewska J, Guzinska-Ustymowicz K, Kemona A. Correlation of c-erbB-2, EGF and EGFR expression with postoperative survival of patients with advanced carcinoma of the stomach. Folia Histochem Cytobiol 2009;47:653-61. [PubMed]
- van Diest P, Budczies J, Klauschen F, et al. Cutoff Finder: A Comprehensive and Straightforward Web Application Enabling Rapid Biomarker Cutoff Optimization. PLoS One 2012;7:e51862 [Crossref] [PubMed]
- Yuan J, Zhang J, Zhu Y, et al. Programmed death-ligand-1 expression in advanced gastric cancer detected with RNA in situ hybridization and its clinical significance. Oncotarget 2016;7:39671-9. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [Crossref] [PubMed]
- Ribeiro J, Oliveira C, Malta M, et al. Epstein-Barr virus gene expression and latency pattern in gastric carcinomas: a systematic review. Future Oncol 2017;13:567-79. [Crossref] [PubMed]
- Zhao J, Liang Q, Cheung KF, et al. Genome-wide identification of Epstein-Barr virus-driven promoter methylation profiles of human genes in gastric cancer cells. Cancer 2013;119:304-12. [Crossref] [PubMed]
- Shinozaki-Ushiku A, Kunita A, Fukayama M. Update on Epstein-Barr virus and gastric cancer Int J Oncol 2015;46:1421-34. (review). [Crossref] [PubMed]
- Liu X, Liu J, Qiu H, et al. Prognostic significance of Epstein-Barr virus infection in gastric cancer: a meta-analysis. BMC Cancer 2015;15:782. [Crossref] [PubMed]
- Gulley ML. Genomic assays for Epstein-Barr virus-positive gastric adenocarcinoma. Exp Mol Med 2015;47:e134 [Crossref] [PubMed]
- Saito R, Abe H, Kunita A, et al. Overexpression and gene amplification of PD-L1 in cancer cells and PD-L1(+) immune cells in Epstein-Barr virus-associated gastric cancer: the prognostic implications. Mod Pathol 2017;30:427-39. [Crossref] [PubMed]
- Ma C, Patel K, Singhi AD, et al. Programmed Death-Ligand 1 Expression Is Common in Gastric Cancer Associated With Epstein-Barr Virus or Microsatellite Instability. Am J Surg Pathol 2016;40:1496-506. [Crossref] [PubMed]
- Seo AN, Kang BW, Kwon OK, et al. Intratumoural PD-L1 expression is associated with worse survival of patients with Epstein-Barr virus-associated gastric cancer. Br J Cancer 2017;117:1753-60. [Crossref] [PubMed]
- Okines A, Cunningham D, Chau I. Targeting the human EGFR family in esophagogastric cancer. Nat Rev Clin Oncol 2011;8:492-503. [Crossref] [PubMed]
- Arteaga CL, Engelman JA. ERBB Receptors: From Oncogene Discovery to Basic Science to Mechanism-Based Cancer Therapeutics. Cancer Cell 2014;25:282-303. [Crossref]
- Gao M, Liang XJ, Zhang ZS, et al. Relationship between expression of EGFR in gastric cancer tissue and clinicopathological features. Asian Pac J Trop Med 2013;6:260-4. [Crossref] [PubMed]
- Zhang H, Deng T, Liu R, et al. Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nat Commun 2017;8:15016. [Crossref] [PubMed]
- Ji M, Liu Y, Li Q, et al. PD-1/PD-L1 expression in non-small-cell lung cancer and its correlation with EGFR/KRAS mutations. Cancer Biol Ther 2016;17:407-13. [Crossref] [PubMed]
- Tang Y, Fang W, Zhang Y, et al. The association between PD-L1 and EGFR status and the prognostic value of PD-L1 in advanced non-small cell lung cancer patients treated with EGFR-TKIs. Oncotarget 2015;6:14209-19. [Crossref] [PubMed]
- Suh KJ, Sung JH, Kim JW, et al. EGFR or HER2 inhibition modulates the tumor microenvironment by suppression of PD-L1 and cytokines release. Oncotarget 2017;8:63901-10. [Crossref] [PubMed]


