
Pathological evaluation of the visceral pleura in the radical pleurectomy/decortication for malignant pleural mesothelioma patients
Introduction
In recent years, radical pleurectomy/decortication (P/D) for surgical treatment of patients with resectable malignant pleural mesothelioma (MPM) has been increasing (1,2). The outcomes of P/D are reported to be comparable to those of extrapleural pneumonectomy (EPP) with no significant difference in survival of patients with MPM (3-11). Macroscopic complete resection (MCR) in any surgical procedure for resectable MPM is critically important (12-14). While both P/D and EPP remove the parietal pleura similarly, P/D removes the visceral pleura with preserving the lung parenchyma. Visceral pleura is excised macroscopically by surgeons, and resected specimens are analyzed histopathologically.
The visceral pleura consists of five-layers: mesothelial and medium subcutaneous layers, elastic plate, connective tissue and basement membrane (15). It is important to evaluate where in the five layers lies the actual dissection plane in P/D. There are no reports evaluating the pathology of the visceral pleura after P/D for patients with MPM. In this study, we examined a pathological assessment of surgically removed visceral pleura in P/D and added survival analyses according to the pathology.
Methods
Clinical characteristics of patients
Visceral pleura of consecutive 25 patients with MPM who received radical P/D under the setting of multimodality treatment consisting of surgery and postoperative chemotherapy at Tokyo Medical and Dental University Hospital between April 2010 and April 2018 were studied. The pathological stage was evaluated based on the IMIG staging system, and histological classification was based on the WHO classification (Table 1). Among these patients, there were 20 cases of epithelioid tumor, 1 of desmoplastic tumor and 4 of biphasic tumor. Nine patients had stage I MPM, one had stage II, 11 had stage III, and 4 had stage IV. Clinical records of all patients were fully documented.
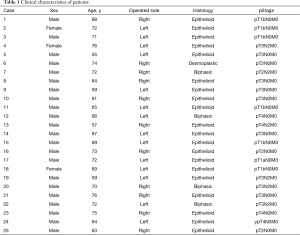
Full table
This study was approved by the Ethics Committee of Tokyo Medical and Dental University (No. M2000-1097), and informed consent was obtained from all the patients.
Surgical procedure
All enrolled patients underwent P/D as their initial surgery. P/D was performed via a wide posterolateral thoracotomy through the sixth rib bed and additional incision at the ninth intercostal space. The parietal pleura was exfoliated radically, and the costal pleural layer was segregated from the endothoracic fascia. All the visceral pleura were removed by a blunt dissection to clear the tumors, leaving the lung parenchyma intact. The diaphragm or pericardium was resected and reconstructed, when the tumor invaded macroscopically. Finally, all patients received intraoperative intrapleural hyperthermic cisplatin perfusion for one hour (2). MCR was obtained in all patients.
Histologic evaluation of visceral pleura
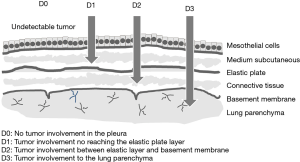
In this study, only visceral pleural lesions were evaluated. Whole removed pleura including adjacent tissue after P/D was fixed in the formalin in the operating room. A few days later, both surgeons and pathologists identified the parietal and visceral pleura together in the pathology lab. At least two specimens of visceral pleura each lobe were obtained for histopathological analysis. We examined a total of 99 lesions in 45 lobes from 25 patients at the following sites: right upper lesions (n=20), middle lesions (n=14), lower lesions (n=20), left upper lesions (n=22), and lower lesions (n=23) (Table 2). Microscopic examinations were made from slides stained with both hematoxylin-eosin (H&E) and Elastica van Gieson (EVG) stains according to standard protocols. The decision to separate the pleura from the parenchyma was based on the macroscopic invasion of the disease. We defined the involvement of the visceral pleura using the depth criteria (Figure 1); type D0: no tumor involvement in the pleura; type D1: tumor involvement is not reaching the elastic plate; type D2: tumor involvement between the elastic plate and the basement membrane and type D3: tumor involvement up to the lung parenchyma. The structures of mesothelial and medium subcutaneous layers, elastic plate, connective tissue, basement membrane, lung parenchyma, and the separation of the pleural surfaces were pathologically evaluated by H&E and EVG staining of the pleura in 99 lesions collected from 25 patients. The stained sections were examined by two authors (M Kobayashi and C Takasaki) without knowledge of the patient characteristics.

Full table
Statistical analysis
We analyzed overall survival (OS) and disease recurrence-free survival (DFS) of patients and compared 2 groups with or without invasion to elastic layer in the visceral pleura. We defined D0&1 as without invasion to elastic layer and D2&3 as with invasion. When different depth criteria were seen in different lobes in a patient, the deepest one was applied. OS was considered as the period from primary surgery to death of the patient or last contact. DFS was considered as the period from primary surgery to diagnosis of first recurrence or last contact. OS and DFS were estimated using the product-limit procedure (Kaplan-Meier method). P<0.05 was considered statistically significant. All statistical analyses were performed using Stat View version 5.0 (SAS Institute Inc.).
Results
Pathological evaluation
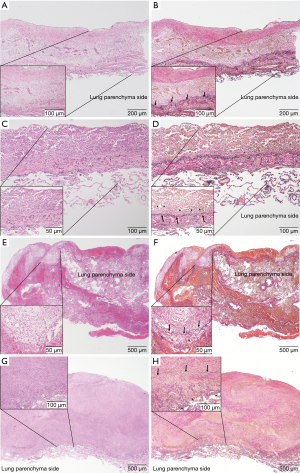
In all the evaluated specimens, the growth of tumor cells on the visceral pleural surface showed either a partially diffuse or nodular pattern. Based on the depth criteria, the lesions were grouped as follows: 21 of type D0 (Figure 2A,B), 14 of type D1 (Figure 2C,D), 22 of type D2 (Figure 2E,F) and 38 of type D3 (Figure 2G,H) (Table 3). Using both HE and EVG staining, histopathological analyses revealed that the dissection plane was the lung parenchyma in all specimens, regardless of tumor involvement. Additionally, the dissection plane of type D3 was deeper in the lung parenchyma compared to that of types D0–2. Therefore, our findings demonstrate that in cases of MPM undergoing P/D, the visceral pleura dissection plane is the lung parenchyma.

Full table
Survivals analysis
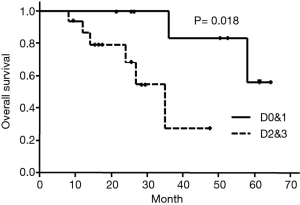
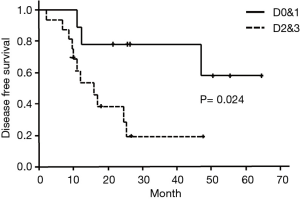
OS in type D0&1 and type D2&3 were shown in Figure 3. DFS in type D0&1 and type D2&3 were shown in Figure 4. Type D0&1 was associated with better OS (P=0.018) and DFS (P=0.024) than type D2&3.
Discussion
It is difficult to evaluate the involvement of the visceral pleura at preoperative. Pinelli et al. assessed the involvement of the visceral pleura by studying the endoscopic patterns of plural invasion in patients with MPM (16). They showed that the cytology of the pleural fluid and the endoscopic appearance were associated with the detection of an advanced stage MPM. It is clear that the prognosis of MPM is related to the extent of visceral pleura involvement independent of parietal pleura involvement (17-19). However, endoscopic examination does not always reveal the extent of visceral pleura invasion. The depth of pleural invasion can be defined only by histopathological investigation.
The essence of P/D is to preserve the lung parenchyma, and it is critical to perform an accurate dissection of the pleural lesions. However, there is no report that evaluates the location of the visceral pleura dissection plane. We established the depth type criteria, to determine the dissection plane in P/D. We scored the tumor involvement based on HE and EVG staining. While the HE staining detects MPM cells in the visceral pleura, the EVG staining evaluates the elastic plate and the basement membrane. A combination of these staining methods helped demonstrate the depth of tumor invasion and dissection plane of the visceral pleurectomy, accurately.
The five-layer structure of the pleura is arranged in the order of mesothelial layer, sub mesothelial layer, elastic plate, connective tissue, and basement membrane (15). MPM cells in the pleural surface extend from the mesothelial layer deep into the lung parenchyma. We evaluated the visceral pleura side in the resected MPMs using the depth criteria. Tumor involvement based on depth was categorized into types D0–3 (Figure 1). As a result, we have shown that the visceral pleura dissection plane was the lung parenchyma regardless of tumor depth. Dissection of the visceral pleura between the tumor and normal was achieved by blunt dissection. Analyses of these findings show that types D0/D1, and D2 has no tumor involvement in the lung parenchyma with some thickening of the mesothelial layer. On the other hand, type D3 was found to have thicker pleura compared to types D0–2, and dissection of the tumor involved going deeper into the lung parenchyma. Therefore, regardless of the tumor, the dissection plane was always within the lung parenchyma.
Decortication of the lung in chronic pleural empyema is performed as a surgical treatment (20-23). Chronic pleural empyema is caused by tuberculosis or other bacterial infection (22). Previous reports have described the dissection plane on the visceral pleura side and reasoned that the visceral pleura remains normal, between the “peel” and the visceral pleura behind the basement membrane (20,23). Pleural thickening occurred with the inflammatory process of dense collagenous fibrous tissue around the pleural mesothelium layer in chronic empyema. On the other hand, pleural thickening in MPM is affected by tumor growth. MPM cells proliferate in visceral pleura, however, dissection plane does not occur within the visceral pleura structure. Malignant disease is potentially different from benign inflammatory diseases
In addition to pathological assessment of visceral pleura, we analyzed the relationship between depth of the tumor invasion and prognosis. Tumor involvement into elastic plate influenced poor prognosis and high recurrence rate. The depth criteria of visceral pleura could help us in classifying pleural invasion histologically and possibly predicting the prognosis.
This study has several limitations. First, it had a small number of cases from a single institution. Future collaborative research using larger sample sizes to obtain an agreement of dissection plane of the procedure. Second, not all of the visceral pleura were microscopically examined in the MPM patients. Third, being a retrospective study, a patient bias could have existed.
Conclusions
In P/D for MPM, the dissection plane of visceral pleura was the lung parenchyma regardless of tumor involvement. Pathological assessment with the depth criteria of pleural invasion would provide a classification of pleural involvement and an estimate of the prognosis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of Tokyo Medical and Dental University (No. M2000-1097), and informed consent was obtained from all the patients.
References
- Flores RM. Surgical options in malignant pleural mesothelioma: extrapleural pneumonectomy or pleurectomy/decortication. Semin Thorac Cardiovasc Surg 2009;21:149-53. [Crossref] [PubMed]
- Ishibashi H, Kobayashi M, Takasaki C, et al. Interim results of pleurectomy/decortication and intraoperative intrapleural hyperthermic cisplatin perfusion for patients with malignant pleural mesothelioma intolerable to extrapleural pneumonectomy. Gen Thorac Cardiovasc Surg 2015;63:395-400. [Crossref] [PubMed]
- Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg 2008;135:620-6. [Crossref] [PubMed]
- Lang-Lazdunski L, Bille A, Lal R, et al. Pleurectomy/decortication is superior to extrapleural pneumonectomy in the multimodality management of patients with malignant pleural mesothelioma. J Thorac Oncol 2012;7:737-43. [Crossref] [PubMed]
- Taioli E, Wolf AS, Flores RM. Meta-analysis of survival after pleurectomy decortication versus extrapleural pneumonectomy in mesothelioma. Ann Thorac Surg 2015;99:472-80. [Crossref] [PubMed]
- Sugarbaker DJ, Wolf AS, Chirieac LR, et al. Clinical and pathological features of three-year survivors of malignant pleural mesothelioma following extrapleural pneumonectomy. Eur J Cardiothorac Surg 2011;40:298-303. [PubMed]
- Krug LM, Pass HI, Rusch VW, et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:3007-13. [Crossref] [PubMed]
- Batirel HF, Metintas M, Caglar HB, et al. Trimodality treatment of malignant pleural mesothelioma. J Thorac Oncol 2008;3:499-504. [Crossref] [PubMed]
- Van Schil PE, Baas P, Gaafar R, et al. Phase II trial of trimodality therapy for malignant pleural mesothelioma (EORTC 08031). Eur Respir J 2010;36:1362-9. [Crossref] [PubMed]
- Trousse DS, Avaro JP, D’journo XB, et al. Is malignant pleural mesothelioma a surgical disease? A review of 83 consecutive extra-pleural pneumonectomies. Eur J Cardiothorac Surg 2009;36:759-63. [Crossref] [PubMed]
- Richards WG, Zellos L, Bueno R, et al. Phase I to II study of pleurectomy/decortication and intraoperative intracavitary hyperthermic cisplatin lavage for mesothelioma. J Clin Oncol 2006;24:1561-7. [Crossref] [PubMed]
- Sugarbaker DJ. Macroscopic complete resection: the goal of primary surgery in multimodality therapy for pleural mesothelioma. J Thorac Oncol 2006;1:175-6. [Crossref] [PubMed]
- Sugarbaker DJ, Garcia JP, Richards WG, et al. Extrapleural pneumonectomy in the multimodality therapy of malignant pleural mesothelioma. Results in 120 consecutive patients. Ann Surg 1996;224:288-94; discussion 294-6. [Crossref] [PubMed]
- Shimokawa M, Hasegawa S, Fukuoka K, et al. A feasibility study of induction pemetrexed plus cisplatin followed by pleurectomy/decortication aimed at macroscopic complete resection for malignant pleural mesothelioma. Jpn J Clin Oncol 2013;43:575-8. [Crossref] [PubMed]
- Osaki T, Nagashima A, Yoshimatsu T, et al. Visceral pleural involvement in nonsmall cell lung cancer: prognostic significance. Ann Thorac Surg 2004;77:1769-73. [Crossref] [PubMed]
- Pinelli V, Laroumagne S, Sakr L, et al. Pleural fluid cytological yield and visceral pleural invasion in patients with epithelioid malignant pleural mesothelioma. J Thorac Oncol 2012;7:595-8. [Crossref] [PubMed]
- Rusch VW. A proposed new international TNM staging system for malignant pleural mesothelioma. From the International Mesothelioma Interest Group. Chest 1995;108:1122-8. [Crossref] [PubMed]
- Boutin C, Rey F, Gouvernet J, et al. Thoracoscopy in pleural malignant mesothelioma: a prospective study of 188 consecutive patients. Part 2: Prognosis and staging. Cancer 1993;72:394-404. [Crossref] [PubMed]
- O’Byrne KJ, Edwards JG, Waller DA. Clinico-pathological and biological prognostic factors in pleural malignant mesothelioma. Lung Cancer 2004;45:S45-8. [Crossref] [PubMed]
- Waller DA, Rengarajan A. Thoracoscopic decortication: a role for video-assisted surgery in chronic postpneumonic pleural empyema. Ann Thorac Surg 2001;71:1813-6. [Crossref] [PubMed]
- Carey JA, Hamilton JRL, Spencer DA, et al. Empyema thoracis: a role for open thoracotomy and decortication. Arch Dis Child 1998;79:510-3. [Crossref] [PubMed]
- Savage T, Fleming HA. Decortication of the lung in tuberculous disease: a study in 43 cases. Thorax 1955;10:293-308. [Crossref] [PubMed]
- Burford TH, Parker EF, Samson PC. Early pulmonary decortication in the treatment of posttraumatic empyema. Ann surg 1945;122:163-90. [Crossref] [PubMed]


