
Building mass to prevent non-alcoholic fatty liver disease?
While increased adiposity, generally estimated by high body mass index (BMI) is the major risk factor for non-alcoholic fatty liver disease (NAFLD), several studies have now reported an association between low skeletal muscle mass and this condition (1,2). In particular, sarcopenia has been associated with NAFLD in the general population, and with disease severity in patients who underwent liver biopsy (3-5).
The pathophysiological mechanisms of sarcopenia, that is of inappropriately low muscle mass, are multifactorial and not fully understood. It remains unclear whether sarcopenia plays a causal role in NAFLD or whether it is a marker of more severe insulin resistance or systemic inflammation. However, sarcopenia and NAFLD share similar mediators. For example, insulin resistance is significantly associated with sarcopenia. Loss of muscle mass reduces a key cellular target for insulin, contributing to glucose intolerance and promoting gluconeogenesis, which exacerbates proteolysis and muscle depletion. Moreover, in adipose tissue insulin resistance results in enhanced lipolysis and availability of free fatty acids, which are taken up by the liver if not by the muscle, ultimately resulting in NAFLD. The association between sarcopenia and NAFLD may also be explained by activation of inflammatory pathways, such as those driven by interleukin-6 and nuclear factor kappa B, that are frequently turned on in NAFLD and that induce protein catabolism (3,6).
Other mechanisms explaining sarcopenia comprise myokine mediators, that are circulating factors with hormone activity released by myocytes. For example, myostatin is a potent negative regulator of skeletal muscle growth, but the myostatin receptor is expressed by hepatic stellate cells. This raises the question as to whether NAFLD-related sarcopenia is caused by myostatin activation in skeletal muscle, or sarcopenia is the primary abnormality related to myostatin, and consequently function in an endocrine manner thus activating fibrogenic hepatic stellate cells. Irisin, the cleaved extra-cellular fragment of the Fibronectin type III domain-containing protein 5 (FNDC5), another exercise-inducible myokine with favorable metabolic activity, is also involved in the control of energy expenditure, body weight, and regulation of insulin resistance (7,8). Recently an association among variants in the FNDC5 gene, in particular rs3480 (G) allele, and severe steatosis and fibrosis has emerged (8,9). Elevated serum irisin was moreover associated with reduced steatosis, and an improved metabolic profile (8). Finally, Growth differentiation factor 15 (GDF15), an endocrine hormone belonging to the TGFβ superfamily, is over-expressed in muscle-wasting conditions. As sarcopenia was independently associated with NASH, circulating GDF15 levels might influence the histological severity of NAFLD by controlling muscle mass (10). Skeletal muscle can thus be considered an endocrine organ, which secretes peptides affecting liver function. It is therefore plausible that sarcopenia plays a causative role in fatty liver through altered secretion of various myokines.
In a recent study, Kim et al. (11) examined in a longitudinal population-based cohort of 2,943 subjects with and 12,624 without baseline NAFLD with a 7-year follow-up whether relative skeletal muscle mass and changes in relative muscle mass were associated with the development or the resolution of this condition. The diagnosis of NAFLD was established according to the hepatic steatosis score (HSI), while skeletal muscle mass was evaluated using bioelectrical impedance analysis and expressed by skeletal muscle mass index (SMI), a measure of body weight-adjusted appendicular skeletal muscle mass. As compared with the lowest tertile, patients in the highest SMI tertile were protected against incident NAFLD [adjusted hazard ratio, AHR =0.44; 95% confidence interval (CI) =0.38–0.51] and more likely to experience resolution of baseline NAFLD (AHR =2.09, 95% CI =1.02–4.28), regardless of sex, age, waist circumference, diabetes, hypertension, smoking status and the level of physical exercise. A novel finding was that subjects in the highest tertile of SMI increase over a year demonstrated a beneficial association with incident NAFLD (AHR =0.69, 95% CI =0.59–0.82) and resolution of baseline NAFLD (AHR =4.17, 95% CI =1.90–6.17), which was also independent of baseline SMI. This observation is important, because it validates the association between muscle mass and protection from liver disease prospectively, and suggest that interventions aimed at increasing SMI may lead to NAFLD resolution.
These data complement those from previous studies reporting similar results (reported in Table 1). In particular, Hong et al. (1) reported in an observational cohort study involving 452 healthy subjects from South Korea an increased risk of NAFLD, as estimated by liver attenuation index at computed tomography (CT) in individuals with lower SMI calculated by dual energy X-ray absorptiometry (DXA). The association between SMI and NAFLD was confirmed by Lee et al. (3), in a cross sectional study in which 15,132 Asian subjects were enrolled. Besides, patients with lower SMI were more likely to have advanced fibrosis estimated by BARD or FIB-4 scores (3). Interestingly the risk of NAFLD was lower in individuals taking regular exercise. In another study (4) evaluating 309 Asiatic subjects with liver histology and bioelectrical impedance analysis (BIA), a higher prevalence of sarcopenia was detected in patients with NAFLD and NASH and there was an inverse correlation between appendicular skeletal muscle mass (ASM) and the severity of fibrosis. Sarcopenia resulted associated with NAFLD (although not independently of metabolic confounders) and with significant fibrosis independent of BMI and insulin resistance. Moreover, patients with reduced muscle mass were more prone to have NASH, regardless of age, gender, BMI, hypertension, diabetes, smoking status and insulin resistance. Petta et al. (5) examined skeletal muscle mass and liver histology in 225 consecutive Western patients with NAFLD. A higher prevalence of sarcopenia in patients with severe fibrosis and an association between sarcopenia and severe steatosis were found, which persisted after adjustment for confounders. A direct association between sarcopenia and NAFLD was confirmed by Choe et al. (2) in a retrospective study involving 1,828 subjects from South Korea evaluated for liver steatosis by ultrasonography and skeletal muscle mass by CT. Nevertheless, after correcting for sex, age, ethnicity, obesity or insulin resistance sarcopenia did not result associated with prevalent NAFLD in 9,985 individuals included in the NHANES-III study, where liver steatosis was investigated by US and skeletal muscle mass by MRI and bioelectrical impedance formula (OR =1.00, 95% CI =0.79–1.27) (12).
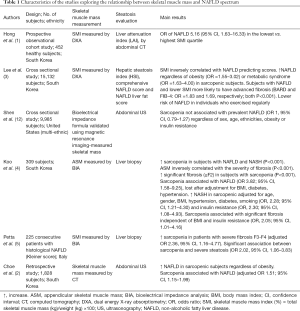
Full table
All in all, the majority of studies seem to suggest a close link between NAFLD and skeletal muscle mass. Limitations however are still to be considered. First of all, quite a large heterogeneity in the definition of NAFLD remains. For instance, the presence and the grade of hepatic steatosis have been evaluated by using different methods (US, CT, liver histology, noninvasive scores) and only two among the works presented refer to liver biopsy for the diagnosis of NAFLD. The same is valid for the evaluation of skeletal muscle mass that has been examined by BIA, DXA or CT. Moreover, the role of physical exercise, namely type, duration, frequency and the possible use of dietary supplementations have not systematically been evaluated. Importantly, a note of caution is necessary regarding patient selection. Because most of the studies enrolled mainly individuals of Asian ethnicity, the conclusions might not be generalizable to other ethnic populations.
Nevertheless, given the significant association between low skeletal muscle mass and NAFLD, and the novel data suggesting that its increase is linked to reduction in indices of hepatic fat accumulation, building muscle mass could be a novel strategy for the prevention and management of this disease. Additional longitudinal and interventional studies evaluating more accurately muscle metabolic and endocrine function together with liver damage are still necessary to confirm these findings, and to identify the best approaches to achieve higher SMI before this approach can be recommended in clinical practice.
Acknowledgements
Funding: L Valenti was supported by myFIRST AIRC grant n.16888 for the EPIDEMIC-NAFLD project (L Valenti), Ricerca Finalizzata 2016 Ministero della Salute - RF-2016-02364358 (L Valenti).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hong HC, Hwang SY, Choi HY, et al. Relationship between sarcopenia and nonalcoholic fatty liver disease: the Korean Sarcopenic Obesity Study. Hepatology 2014;59:1772-8. [Crossref] [PubMed]
- Choe EK, Kang HY, Park B, et al. The Association between Nonalcoholic Fatty Liver Disease and CT-Measured Skeletal Muscle Mass. J Clin Med 2018;7:310. [Crossref] [PubMed]
- Lee YH, Jung KS, Kim SU, et al. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: Nationwide surveys (KNHANES 2008-2011). J Hepatol 2015;63:486-93. [Crossref] [PubMed]
- Koo BK, Kim D, Joo SK, et al. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol 2017;66:123-31. [Crossref] [PubMed]
- Petta S, Ciminnisi S, Di Marco V, et al. Sarcopenia is associated with severe liver fibrosis in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2017;45:510-8. [Crossref] [PubMed]
- Bhanji RA, Narayanan P, Allen AM, et al. Sarcopenia in hiding: The risk and consequence of underestimating muscle dysfunction in nonalcoholic steatohepatitis. Hepatology 2017;66:2055-65. [Crossref] [PubMed]
- Zhai Y, Xiao Q. The Common Mechanisms of Sarcopenia and NAFLD. Biomed Res Int 2017;2017:6297651. [Crossref] [PubMed]
- Metwally M, Bayoumi A, Romero-Gomez M, et al. A polymorphism in the Irisin-encoding gene (FNDC5) associates with hepatic steatosis by differential miRNA binding to the 3'UTR. J Hepatol 2019;70:494-500. [Crossref] [PubMed]
- Petta S, Valenti L, Svegliati-Baroni G, et al. Fibronectin type III domain-containing protein 5 rs3480 A>G polymorphism, irisin, and liver fibrosis in patients with Nonalcoholic fatty liver disease. J Clin Endocrinol Metab 2017;102:2660-9. [Crossref] [PubMed]
- Koo BK, Um SH, Seo DS, et al. Growth differentiation factor 15 predicts advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease. Liver Int 2018;38:695-705. [Crossref] [PubMed]
- Kim G, Lee SE, Lee YB, et al. Relationship Between Relative Skeletal Muscle Mass and Nonalcoholic Fatty Liver Disease: A 7-Year Longitudinal Study. Hepatology 2018;68:1755-68. [Crossref] [PubMed]
- Shen H, Liangpungsakul S. Association between sarcopenia and prevalence of nonalcoholic fatty liver disease: a cross-sectional study from the third National health and nutrition examination survey. Gastroenterology 2016;150:S1143-S1144. [Crossref]


