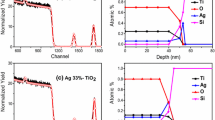
Abstract
Ag nanoparticles dispersed SiO2 composite films were successfully prepared by a sol-gel method. The structural transition, formation, and optical property along with relevant band gap of Ag/SiO2 thin films during the annealing process were studied by Fourier transform infrared spectroscopy, thermogravimetry-differential thermal analysis, x-ray diffraction, and ultraviolet-visible spectroscopy, while the micro structure of thin films was revealed by transmission electron microscopy. The results indicate that the Ag spherical particles with the diameter of 10-20 nm were formed by breaking Si-O-Ag bonds above 200 °C and dispersed in the SiO2 matrix. The optical absorption property of Ag/SiO2 nanofilm in the visible range is enhanced, and the band gap (Eg) is widened with raising annealing temperatures, which is promising for the potential applications in nonlinear optical and related fields.







Similar content being viewed by others
References
U. Kreibig and M. Vollmer, Optical Properties of Metal Clusters (Springer-Verlag, Berlin, Germany, 1995).
C. Sella, S. Chenot, V. Reillon, and S. Berthier: Influence of the deposition conditions on the optical absorption of Ag-SiO2 nano-cermet thin films. Thin Solid Films 517, 5848 (2009).
U. Kreibig and L. Genzel: Optical absorption of small metallic particles. Surf. Sci. 156, 678 (1985).
B.J. Persson: Surface resistivity and vibrational damping in adsorbed layers. Phys. Rev. B 44, 3277 (1991).
G.D. Stucky and J.E. Mac Dougall: Quantum confinement and host/guest chemistry: Probing a new dimension. Science 247(4943), 669 (1990).
A.A. Scalisi, G. Compagnini, L. D’Urso, and O. Puglisi: Nonlinear optical activity in Ag-SiO2 nanocomposite thin films with different silver concentration. Appl. Surf. Sci. 226, 237 (2004).
L. Guo, A. Guan, X. Lin, C. Zhang, and G. Chen: Preparation of a new core-shell Ag@SiO2 nanocomposite and its application for fluorescence enhancement. Talanta 82, 1696 (2010).
P. Gangopadhyay, R. Kesavamoorthy, K.G.M. Nair, and R. Dhandapani: Raman scattering studies on silver nanoclusters in a silica matrix formed by ion-beam mixing. J. Appl. Phys. 88(9), 4975 (2000).
Z.X. Liu, H. Li, X.D. Feng, S.G. Ren, and H.H. Wang: Formation effects and optical absorption of Ag nanocrystals embedded in single crystal SiO2 by implantation. J. Appl. Phys. 84(4), 1913 (1998).
L. Yang, Y.L. Liu, Q.M. Wang, H.Z. Shi, G.H. Li, and L.D. Zhang: The plasmon resonance absorption of Ag/SiO2 nanocomposite films. Microelectron. Eng. 66, 192 (2003).
I. Tanahashi, M. Yoshida, Y. Manabe, and T. Tohda: Effects of heat treatment on Ag particle growth and optical properties in Ag/SiO2 glass composite thin films. J. Mater. Res. 10, 362 (1995).
A. Babapour, O. Akhavan, A.Z. Moshfegh, and A.A. Hosseini: Size variation and optical absorption of sol-gel Ag nanoparticles doped SiO2 thin film. Thin Solid Films 515, 771 (2006).
L.S. Jiao, B.P. Zhang, X.Z. Ding, C. Chen, and H.L. Zhang: Sol-gel preparation of Ag/SiO2 nano-composite films and their optical absorption properties. Rare Met. Mater. Eng. 36, 882 (2007).
M. Chatterjee and M.K. Naskar: Sol-gel synthesis of lithium aluminum silicate powders: The effect of silica source. Ceram. Int. 32, 623 (2006).
E. Monsivais-Gamez, F. Ruiz, and J.R. Martinez: Four-membered rings family in the Si-O extended rocking IR band from quantum chemistry calculations. J. Sol-Gel Sci. Technol. 43(1), 65 (2007).
V.K. Parashar, V. Raman, and O.P. Bahl: The role of N,N, dimethylformamide and glycol in the preparation and properties of sol-gel derived silica. J. Mater. Sci. Lett. 15(16), 1403 (1996).
M. Stefanescu, M. Stoia, and O. Stefanescu: Thermal and FT-IR study of the hybrid ethylene-glycol-silica matrix. J. Sol-Gel Sci. Technol. 41(1), 71 (2007).
W.X. Que, Y. Zhou, Y.L. Lam, Y.C. Chan, H.T. Tan, T.H. Tan, and C.H. Kam: Sol-gel processed silica/titania/y-glycidoxypropy-ltrimethoxysilane composite materials for photonic applications. J. Electron. Mater. 29(8), 1052 (2000).
N.B. Colthup, L.H. Daly, and S.E. Wiberiey: Introduction to Infrared and Raman Spectroscopy, 2nd ed (Academic Press, New York, 1975).
J. Soderlund, L.B. Kiss, G.A. Niklasson, and C.G. Granqvist: Lognormal size distributions in particle growth processes without coagulation. Phys. Rev. Lett. 80(11), 2386 (1998).
B. Karmakar, G. De, and D. Ganguli: Dense silica microspheres from organic and inorganic acid hydrolysis of TEOS. J. Non-Cryst. Solids 272(40239), 119 (2000).
G. De, B. Karmakar, and D. Ganguli: Hydrolysis-condensation reactions of TEOS in presence of acetic acid leading to the generation of glass-like silica microspheres in solution at room temperature. J. Mater. Chem. 10, 2289 (2000).
G. De, D. Kundu, B. Karmakar, and D. Ganguli: FTIR studies of gel to glass conversion in TEOS-fumed silica derived gels, J. Non-Cryst. Solids 155, 253 (1993).
H.J. Jeon, S.C. Yi, and S.G. Oh: Preparation and antibacterial effects of Ag-SiO2 thin films by sol-gel method. Biomaterials 24, 4921 (2003).
J. Wang, C.R. Zhang, and J. Feng: Modification of nanoporous silica film by trimethylchlorosilane. Acta Phys. Chim. Sin. 20, 1399 (2004).
P. Innocenzi: Infrared spectroscopy of sol-gel derived silica-based films: A spectra-microstructure overview. J. Non-Cryst. Solids 316(2-3), 309 (2003).
J.R. Martinez, F. Ruiz, Y.V. Vorobiev, F. Pérez-Robles, and J. Gonzalez-Hernandez: Infrared spectroscopy analysis of the local atomic structure in silica prepared by sol-gel. J. Chem. Phys. 109(17), 7511 (1998).
M. Parler Caroline, A. Ritter James, and D. Amiridis Michael: Infrared spectroscopic study of sol-gel derived mixed-metal oxides, J. Non-Cryst. Solids 279(2-3), 119 (2001).
D. Niznansky and J.L. Rehspringer: Infrared study of SiO2 sol to gel evolution and gel aging. J. Non-Cryst. Solids 180, 191 (1995).
M. Yamane: in Sol-Gel Technology for Thin Films, edited by L.C. Klein (Noyes Publications, New Jersey, 1989).
C.C. Perry, X. Li, and D.N. Waters: Structural studies of gel phases. 4. An infrared reflectance and Fourier-transform Raman-study of silica and silica titania gel glasses. Spectrochim. Acta 47, 1487 (1991).
L. Lan, G. Gnappi, and A. Montenero: Infrared study of EPOXS-TEOS-TPOT gels. J. Mater. Sci. 28, 2119 (1993).
C.H. Zhao, B.P. Zhang, and P.P. Shang: Enhanced nonlinear optical absorption of Au/SiO2 nano composite thin films. Chin. Phys. B 18, 5539 (2009).
R. Pinkos, M. Wesotowski, and J. Teodorczyk: Thermal analysis of some pharmaceutically relevant systems obtained by sol-gel technique. J. Therm. Anal. Calorim. 70, 447 (2002).
L.H. Allen and E. Matijevic: Stability of colloidal silica: III. Effect of hydrolyzable cations. Interface Sci. 35, 66 (1971).
Acknowledgments
The authors thank Mr. Xi-Zhen Ding (graduate student of USTB) for a part of his experiments. This work was supported by Major State Basic Research Development (Grant No. 2007CB613301), the National Natural Science Foundation of China (Grant No. 50972012), and Specialized Research Fund for the Doctoral Program of Higher Education (Grant No. 20090006110010).
Author information
Authors and Affiliations
Corresponding author
Additional information
Address all correspondence to this author.
Rights and permissions
About this article
Cite this article
Li, Y., Zhang, BP., Zhao, CH. et al. Structure transition, formation, and optical absorption property study of Ag/SiO2 nanofilm by sol-gel method. Journal of Materials Research 27, 3141–3146 (2012). https://doi.org/10.1557/jmr.2012.388
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2012.388