Abstract
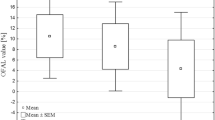
Little is known about the genetic alterations that occur during the progression of thyroid neoplasms. To understand better the biology of thyroid tumors, we investigated several genetic loci in benign and malignant thyroid neoplasms. Forty-one thyroid tumors (6 adenomas, 16 papillary, 14 follicular, and 5 anaplastic carcinomas) were studied. Normal and tumor cells were microdissected from paraffin-embedded tissues. DNA was used for polymerase chain reaction-based loss of heterozygosity (LOH) analysis with the following markers: D1S243 (1p35–36), D1S165 (1p36) and D1S162 (1p32), TP53 (17p13), and INT-2 (11q13). Immunohistochemistry for Ki-67 was performed. The Ki-67 labeling index (LI) was the percentage of positive tumor cells. LOH at 1p was seen in 2 of 5 (40%) informative cases of anaplastic carcinoma (2 of 2 at D1S162 and 1 of 2 at D1S165) and in 2 of 11 (18%) informative cases of follicular carcinoma (2 of 7 at D1S243, 2 of 7 at D1S165, and 1 of 6 at D1S162). One anaplastic (20%) and two follicular carcinomas (14%) had LOH in at least two of the 1p loci analyzed. None of the adenomas and papillary carcinomas had LOH at these loci. LOH at 17p and 11q13 were infrequent. Ki-67 LI was 1.4, 7, 16, and 65% in adenomas, papillary, follicular, and anaplastic carcinomas, respectively. Allelic loss at 1p may occur in aggressive types of thyroid carcinoma and may be a marker of poor prognosis. LOH at 1p may represent a late genetic event in thyroid carcinogenesis. LOH at 17p and 11q13 (MEN gene locus) is uncommon in thyroid neoplasms.
Similar content being viewed by others
References
Fagin JA, Matsuo K, Karmakar A, Chen DL, Tang SH, and Koeffler HP. High prevalence of mutations of the p53 gene in poorly differentiated human thyroid carcinomas. J Clin Invest 91:179–184,1993.
Santoro M, Carlomagno F, Hay ID, et al. Ret oncogene activation in human thyroid neoplasms is restricted to the papillary subtype. J Clin Invest 89:1517–122,1992.
Santoro M, Grieco M, Melillo RM, Fusco A, Vecchio G. Molecular defects in thyroid carcinomas: role of the RET oncogene in thyroid neoplastic transformation. Eur J Endocrinol 133:513–522,1995.
Fusco A, Grieco M, Santoro M, et al. A new oncogene in human thyroid papillary carcinomas and their lymph-nodal metastases. Nature 328:170–173,1987.
Namba H, Rubin SA, Fagin JA. Point mutations of ras oncogenes are an early event in thyroid tumorigenesis. Mol Endocrinol 4:1474–1479,1990.
Suarez HG, du Villard JA, Severino M, et al. Presence of mutations in all three ras genes in human thyroid tumors. Oncogene 5:565–570,1988.
Grieco M, Santoro M, Berlingieri MT, et al. PTC is a novel rearranged form of the ret proto-oncogene and is frequently detected in vivo in human thyroid papillary carcinomas. Cell 60:557–563,1990.
Salvatore D, Celetti A, Fabien N, et al. Low frequency of p53 mutations in human thyroid tumors; p53 and Ras mutation in two out of fifty-six thyroid tumours. Eur J Endocrinol 134:177–183,1996.
Ito T, Seyama T, Mizuno T, et al. Unique association of p53 mutations with undifferentiated but not with differentiated carcinomas of the thyroid gland. Cancer Res 52:1369–1371,1992.
Pilotti S, Collini P, Del Bo R, Cattoretti G, Pierotti MA, Rilke F. A novel panel of antibodies that segregates immunocytochemically poorly differentiated carcinoma from undifferentiated carcinoma of the thyroid gland. Am J Surg Pathol 18(10):1054–1064,1994.
Grebe SK, McIver B, Hay ID, et al. Frequent loss of heterozygosity on chromosomes 3p and 17p without VHL or p53 mutations suggests involvement of unidentified tumor suppressor genes in follicular thyroid carcinoma. J Clin Endocrinol Metab 82:3684–3691,1997.
Amler LC, Corvi R, Praml C, et al. A reciprocal translocation (1;15)(36.2;q24) in a neuroblastoma cell line is accompanied by DNA duplication and may signal the site of a putative tumor suppressor gene. Oncogene 10:1095–1101,1995.
Bardi G, Pandis N, Fenger C, Kronborg O, Bomme L, Heim S. Deletions in 1p36 as a primary chromosomal aberration in intestinal tumorigenesis. Cancer Res 53:1895–1898,1993.
Cheng NC, Van Roy N, Chan A, Beitsma M, et al. Deletion mapping in neuroblastoma cell lines suggests two distinct tumor suppressor genes in the 1p35–36 region only one of which is associated with N-myc. Oncogene 10:291–297,1995.
Leister I, Weith A, Bruderlein S, et al. Human colorectal cancer: high frequency of deletions at chromosome 1p35. Cancer Res 50:7232–7235,1990.
Rosai J, Carcangiu M.L, DeLellis R.A. Tumors of the thyroid gland. In: Rosai, J, ed. Atlas of tumor pathology, 3rd series. Washington, DC: Armed Forces Institute of Pathology, 1992.
Zhuang Z, Berttheau P, Emmert-Buck MR, Liotta LA, Gnarra J, Linehan WM, Lubensky IA. A new microdissection technique for archival DNA analysis of specific cell populations in lesions less than one millimeter. Am J Pathol 146:620–625,1990.
Jhiang SM, Mazzaferri E.L. The Ret/PTC oncogene in papillary thyroid carcinoma. J Lab Clin Med 123:331–337,1994.
Jhiang SM, Caruso DR, Gilmore E, et al. Detection of the PTC/ret TPC oncogene in human thyroid carcinomas. Oncogene 7:1331–1337,1992.
Bongarzone I, Pierotti MA, Monzini N, et al. High frequency of activation of tyrosine kinase oncogenes in human papillary thyroid carcinoma. Oncogene 4:1457–1462,1989.
Takahashi M. Oncogenic activation of the ret protooncogene in thyroid cancer. Crit. Rev. Oncogenesis 6:35–46,1995.
Namba H, Gutman RA, Matsuo K, Alvarez A, Fagin JA. H-ras proto-oncogene mutations in human thyroid neoplasms. J Clin Endocrinol Metab 71:223–229,1990.
Dobashi Y, Sakamoto A, Sugiura H, et al. Overexpression of p53 as a prognostic factor in human thyroid carcinoma. Am J Surg Pathol 17:375–381,1993.
Harris CC, Hollstein M. Clinical implications of the p53 tumor-suppressor gene. N Engl J Med 329:1318–1327,1993.
Lane DP. p53 and human cancers. Br Med Bull 50:582–599,1994.
Nakamura T, Yana I, Kobayashi T, et al. p53 gene mutations associated with anaplastic transformation of human thyroid carcinomas. Jpn J Cancer Res 83:1293–1298,1992.
Ng IOL, Na J, Lai ECS, Fan ST, Ng M. Ki-67 antigen expression in hepatocellular carcinoma using monoclonal antibody MIB1. Am J Clin Pathol 104:313–318,1995.
Stravropoulos NE, Ioackim-Velogianni E, Hastazeris K, et al. Growth factors in bladder cancer defined by Ki-67: association with cancer grade category and recurrence rate of superficial lesions. Br J Urol 72:736–739,1993.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kleer, C.G., Bryant, B.R., Giordano, T.J. et al. Genetic changes in chromosomes 1p and 17p in thyroid cancer progression. Endocr Pathol 11, 137–143 (2000). https://doi.org/10.1385/EP:11:2:137
Issue Date:
DOI: https://doi.org/10.1385/EP:11:2:137