Abstract
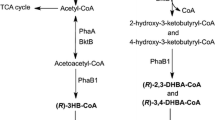
An efficient system for the production of (R)-hydroxyalkanoicacids (RHAs) was developed in natural polyhydroxyalkanoate (PHA)-producing bacteria and recombinant Escherichia coli. Acidic alcoholysis of purified PHA and in vivo depolymerization of PHA accumulated in the cells allowed the production of RHAs. In recombinant E. coli, RHA production was achieved by removing CoA from (R)-3-hydroxyacyl-CoA and by in vivo depolymerization of PHA. When the recombinant E. coli harboring the Ralstonia eutropha PHA biosynthesis genes and the depolymerase gene was cultured in a complex or a chemically defined medium containing glucose, (R)-3-hydroxybutyric acid (R3HB) was produced as monomers and dimers. R3HB dimers could be efficiently converted to monomers by mild alkaline heat treatment. A stable recombinant E. coli strain in which the R. eutropha PHA biosynthesis genes were integrated into the chromosome disrupting the pta gene was constructed and examined for the production of R3HB. When the R. eutropha intracellular depolymerase gene was expressed by using a stable plasmid containing the hok/sok locus of plasmid R1, R3HB could be efficiently produced.
Similar content being viewed by others
References
Anderson, A. J. and Dawes E. A. (1990), Microbiol. Rev. 54, 450–472.
Doi, Y. (1990), Microbial Polyesters, VCH, New York, NY.
Lee, S. Y. (1996), Biotechnol. Bioeng. 49, 1–14.
Steinbüchel, A. and Fuchtenbusch, B. (1998), Trends Biotechnol. 16, 419–427.
Steinbüchel, A. and Valentin, H. E. (1995), FEMS Microbiol. Lett. 128, 219–228.
Chiba, T. and Nakai, T. (1985), Chem. Lett., 651–654.
Schnurrenberger, P., Hungerbühler, E., and Seebach, D. (1987), Liebigs Ann. Chem., 733–744.
Yu, D., Ellis, H. M., Lee, E.-C., Jenkins, N. A., Copeland, N. G., and Court, D. L. (2000), Proc. Natl. Acad. Sci. USA 97, 5978–5983.
Lee, Y., Park, S. H., Lim, I. T., Han, K. and Lee, S. Y. (2000), Enzyme Microb. Technol. 27, 33–36.
Seebach, D., Beck, A. K., Breitschuh, R., and Job, K. (1992), Org. Synth. 71 39–47.
Seebach, D. and Zuger, M. F. (1982), Helvetica Chim. Acta. 65, 495–503.
Peoples, O. P. and Sinskey, A. J. (1989), J. Biol. Chem. 264, 15,298–15,303.
Schubert, P., Steinbüchel, A., and Schlegel, H. G. (1988), J. Bacteriol. 170, 5837–5847.
Slater, S. C., Voige, W. H., and Dennis, D. (1988), J. Bacteriol. 170, 4431–4436.
Lee, S. Y., Lee, Y., and Wang, F. (1999), Biotechnol. Bioeng. 65, 363–368.
Gao, H. J., Wu, Q., and Chen, G. Q. (2002), FEMS Microbiol. Lett. 213, 59–65.
Zhao, K., Tian, G., Zheng, Z., Chen, J. C., and Chen, G. Q. (2003), FEMS Microbiol. Lett. 218, 59–64.
Lee, S. Y. and Lee, Y. (2003), Appl. Environ. Microbiol. 69, 3421–3426.
Hasegawa, J., Ogura, M., Kanama, H., Noda, N., Kawaharada, H., and Watanabe, K.. (1982), J. Ferment. Technol. 60, 501–508.
Deol, B. S., Ridley, D. D., and Simpson, G. W., (1976), Aust. J. Chem. 29, 2459–2467.
Mochizuki, N., Sugai, T., and Ohta, H. (1994), Biosci. Biotech. Biochem. 58, 1666–1670
de Roo, G., Kellerhals, M. B., Ren, Q., Witholt, B., and Kessler, B. (2002), Biotechnol. Bioeng. 77, 717–722.
Choi, J., and Lee, S. Y. (1999), Biotechnol. Bioeng. 62, 546–553.
Madison, L. L. and Huisman, G. W. (1999), Microbiol. Mol. Biol. Rev. 63, 21–53.
Saegusa, H., Shiraki, M., Kanai, C. and Saito, T. (2001), J. Bacteriol. 183, 94–100.
Miltenberger, K. and Aktiengesellschaft, H. (1985–1996), in Ullmann's Encyclopedia of Industrial Chemistry, 5th Ed., Arpe, H.-J. et al., eds., Wiley-VCH, Weinheim, Berlin, Germany, pp. 507–517.
Gerdes, K. (1988), Bio/Technology 6, 1402–1405.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, S.J., Lee, S.Y. & Lee, Y. Biosynthesis of (R)-3-hydroxyalkanoic acids by metabolically engineered Escherichia coli . Appl Biochem Biotechnol 114, 373–379 (2004). https://doi.org/10.1385/ABAB:114:1-3:373
Issue Date:
DOI: https://doi.org/10.1385/ABAB:114:1-3:373