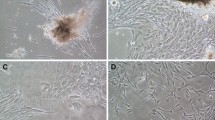
Summary
An immortalized human prostate stromal cell line (PS30) was previously established using recombinant retrovirus encoding human papillomavirus 16 gene products. In this study, we further characterize this stromal cell line for its potential use in a stromal-epithelial coculture model for prostate cancer prevention. Using reverse transcriptase-polymerase chain reaction, enzyme-linked immunosorbent assay, and immunocytochemistry, we examined expression of androgen receptor (AR), vitamin D receptor (VDR), prostate-specific antigen (PSA), transforming growth factor-β (TGF-β), and insulin-like growth factors (IGF) families and their receptors, metalloproteinases (MMP) MMP-2 and MMP-9, as well as the cells' ability to respond to the synthetic androgen R1881. The PS30 stromal cells do not express PSA, confirming their stromal origin. They are positive for both AR messenger ribonucleic acid (mRNA) and protein; however, they do not respond to growth stimulation by the synthetic androgen R1881. The PS30 cells express mRNA for VDR, TGF-βs, IGFs and their receptors, as well as the MMPs. Moreover, they produce significant amounts of TGF-β1, TGF-β2, IGFBP-3, and MMP-2 proteins. Our observations confirm the use of PS30 for the study of stromal-epithelial interactions in the modulation of prostate carcinogenesis.
Similar content being viewed by others
References
Baker, T.; Tickle, S.; Wasan, H.; Docherty, A.; Isenberg, D.; Waxman, J. Serum metalloproteinases and their inhibitors: markers for malignant potential. Br. J. Cancer 70:506–512; 1994.
Barsony, J.; Renyi, I.; McKoy, W. Subcellular distribution of normal and mutant vitamin D receptors in living cells. Studies with a novel fluorescent ligand. J. Biol. Chem. 272:5774–5782; 1997.
Bello-DeOcampo, D.; Tindall, D. J. TGF-betal/Smad signaling in prostate cancer. Curr. Drug Targets 4:197–207; 2003.
Boudon, C.; Rodier, G.; Lechevallier, E.; Mottet, N.; Barenton, B.; Sultan, C. Secretion of insulin-like growth factors and their binding proteins by human normal and hyperplastic prostatic cells in primary culture. J. Clin. Endocrinol. Metab. 81:612–617; 1996.
Byrne, R. L.; Leung, H.; Neal, D. E. Peptide growth factors in the prostate as mediators of stromal epithelial interaction. Br. J. Urol. 77:627–633; 1996.
Cardillo, M. R.; Monti, S.; Di Silverio, F.; Gentile, V.; Sciarra, F.; Toscano, V. Insulin-like growth factor (IGF)-I, IGF-II and IGF type I receptor (IGFR-I) expression in prostatic cancer. Anticancer Res. 23:3825–3835; 2003.
Cardillo, M. R.; Petrangeli, E.; Salvatori, L.; Ravenna, L.; Di Silverio, F. Transforming growth factor beta 1 and androgen receptors in prostate neoplasia. Anal. Quant. Cytol. Histol. 22:403–410; 2000.
Cheifetz, S.; Hernandez, H.; Laiho, M.; ten Dijke, P.; Iwata, K. K.; Massague, J. Distinct transforming growth factor-beta (TGF-beta) receptor subsets as determinants of cellular responsiveness to three TGF-beta isoforms. J. Biol. Chem. 265:20533–20538; 1990.
Christakos, S.; Raval-Pandya, M.; Wernyj, R. P.; Yang, W. Genomic mechanisms involved in the pleiotropic actions of 1,25-dihydroxyvitamin D3. Biochem. J. 316:361–371; 1996.
Chung, L. W. Implications of stromal-epithelial interaction in human prostate cancer growth, progression and differentiation. Semin. Cancer Biol. 4:183–192; 1993.
Cohen, P.; Peehl, D. M.; Lamson, G.; Rosenfeld, R. G. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins in primary cultures of prostate epithelial cells. J. Clin. Endocrinol. Metab. 73:401–407; 1991.
Cunha, G. R.; Hayward, S. W.; Dahiya, R.; Foster, B. A. Smooth muscle-epithelial interactions in normal and neoplastic prostatic development. Acta Anat. (Basel) 155:63–72; 1996.
Cunha, G. R.; Hayward, S. W.; Wang, Y. Z. Role of stroma in carcinogenesis of the prostate. Differentiation 70:473–485; 2002.
Curran, S.; Murray, G. I. Matrix metalloproteinases in tumour invasion and metastasis. J. Pathol. 189:300–308; 2003.
Daja, M. M.; Niu, X.; Zhao, Z.; Brown, J. M.; Russell, P. J. Characterization of expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in prostate cancer cell lines. Prostate Cancer Prostatic Dis. 6:15–26; 2003.
Djakiew, D. Dysregulated expression of growth factors and their receptors in the development of prostate cancer. Prostate 42:150–160; 2000.
Djakiew, D.; Tarkington, M. A.; Lynch, J. H. Paracrine stimulation of polarized secretion from monolayers of a neoplastic prostatic epithelial cell line by prostatic stromal cell proteins. Cancer Res. 50:1966–1974; 1990.
Djavan, B.; Waldert, M.; Seitz, C.; Marberger, M. Insulin-like growth factors and prostate cancer. World J. Urol. 19:225–233; 2001.
Dong, Z.; Nemeth, J. A.; Cher, M. L.; Palmer, K. C.; Bright, R. C.; Fridman, R. Differential regulation of matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-1 (TIMP-1) and TIMP-2 expression in cocultures of prostate cancer and stromal cells. Int. J. Cancer 93:507–515; 2001.
Fowlkes, J. L.; Thrailkill, K. M.; Serra, D. M.; Suzuki, K.; Nagase, H. Matrix metalloproteinases as insulin-like growth factor binding protein-degrading proteinases. Prog. Growth Factor Res. 6:255–263; 1995.
Franks, L. M.; Riddle, P. N.; Carbonell, A. W.; Gey, G. O. A comparative study of the ultrastructure and lack of growth capacity of adult human prostate epithelium mechanically separated from its stroma. J. Pathol. 100:113–119; 1970.
Gerdes, M. J.; Dang, T. D.; Larsen, M.; Rowley, D. R. Transforming growth factor-beta1 induces nuclear to cytoplasmic distribution of androgen receptor and inhibits androgen response in prostate smooth muscle cells. Endocrinology 139:3569–3577; 1998.
Gerdes, M. J.; Larsen, M.; Dang, T. D.; Ressler, S. J.; Tuxhorn, J. A.; Rowley, D. R. Regulation of rat prostate stromal cell myodifferentiation by androgen and TGF-beta1. Prostate 58:299–307; 2004.
Grimberg, A.; Cohen, P. Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J. Cell Physiol. 183:1–9; 2000.
Hamdy, F. C.; Fadlon, E. J.; Cottam, D., et al. Matrix metalloproteinase 9 expression in primary human prostatic adenocarcinoma and benign prostatic hyperplasia. Br. J. Cancer 69:177–182; 1994.
Itoh, N.; Patel, U.; Cupp, A. S.; Skinner, M. K. Developmental and hormonal regulation of transforming growth factor-beta1 (TGFbeta1),-2, and-3 gene expression in isolated prostatic epithelial and stromal cells: epidermal growth factor and TGFbeta interactions. Endocrinology 139:1378–1388; 1998.
Jakowlew, S. B.; Moody, T. W.; Mariano, J. M. Transforming growth factor-beta receptors in human cancer cell lines: analysis of transcript, protein and proliferation. Anticancer Res. 17:1849–1860; 1997.
Jenster, G. The role of the androgen receptor in the development and progression of prostate cancer. Semin. Oncol. 26:407–421; 1999.
Kivineva, M.; Blauer, M.; Syvala, H.; Tammela, T.; Tuohimaa, P. Localization of 1,25-dihydroxyvitamin D3 receptor (VDR) expression in human prostate. J. Steroid Biochem. Mol. Biol. 66:121–127; 1998.
Klingler, H. C.; Bretland, A. J.; Reid, S. V.; Chapple, C. R.; Eaton, C. L. Regulation of prostatic stromal cell growth and function by transforming growth factor beta (TGFbeta). Prostate 41:110–120; 1999.
Krill, D.; DeFlavia, P.; Dhir, R.; Luo, J.; Becich, M. J.; Lehman, E.; Getzenberg, R. H. Expression patterns of vitamin D receptor in human prostate. J. Cell. Biochem. 82:566–572; 2001.
Lee, D. K.; Chang, C. Endocrine mechanisms of disease: expression and degradation of androgen receptor: mechanism and clinical implication. J. Clin. Endocrinol. Metab. 88:4043–4054; 2003.
Lynch, C. C.; Matrisian, L. M. Matrix metalloproteinases in tumor-host cell communication. Differentiation 70:561–573; 2002.
Massague, J. The transforming growth factor-beta family. Annu. Rev. Cell Biol. 6:597–641; 1990.
Miller, G. J.; Stapleton, G. E.; Hedlund, T. E.; Moffat, K. A. Vitamin D receptor expression, 24-hydroxylase activity, and inhibition of growth by 1alpha,25-dihydroxyvitamin D3 in seven human prostatic carcinoma cell lines. Clin. Cancer Res. 1:997–1003; 1995.
Ohtani, H. Stromal reaction in cancer tissue: pathophysiologic significance of the expression of matrix-degrading enzymes in relation to matrix turnover and immune/inflammatory reactions. Pathol. Int. 48:1–9; 1998.
Peehl, D. M.; Cohen, P.; Rosenfeld, R. G. The insulin-like growth factor system in the prostate. World J. Urol. 13:306–311; 1995.
Peehl, D. M.; Skowronski, R. J.; Leung, G. K.; Wong, S. T.; Stamey, T. A.; Feldman, D. Antiproliferative effects of 1,25-dihydroxyvitamin D3 on primary cultures of human prostatic cells. Cancer Res. 54:805–810; 1994.
Pike, J. W. Vitamin D3 receptors: structure and function in transcription. Annu. Rev. Nutr. 11:189–216; 1991.
Planz, B.; Kirley, S. D.; Wang, Q.; Tabatabaei, S.; Aretz, H. T.; McDougal, W. S. Characterization of a stromal cell model of the human benign and malignant prostate from explant culture. J. Urol. 161:1329–1336; 1999.
Price, D. T.; Rudner, X.; Michelotti, G. A.; Schwinn, D. A. Immortalization of a human prostate stromal cell line using a recombinant retroviral approach. J. Urol. 164:2145–2150; 2000.
Roberson, K. M.; Edwards, D. W.; Chang, G. C.; Robertson, C. N. Isolation and characterization of a novel human prostatic stromal cell culture: DuK50. In Vitro Cell. Dev. Biol. 31A:840–845; 1995.
Rowley, D. R. What might a stromal response mean to prostate cancer progression? Cancer Metastasis Rev. 17:411–419; 1999.
Royuela, M.; De Miguel, M. P.; Bethencourt, F. R.; Sanchez-Chapado, M.; Fraile, B.; Paniagua, R. Transforming growth factor beta 1 and its receptor types I and II. Comparison in human normal prostate, benign prostatic hyperplasia, and prostatic carcinoma. Growth Factors 16:101–110; 1998.
Ruizeveld de Winter, J. A.; Janssen, P. J.; Sleddens, H. M., et al. Androgen receptor status in localized and locally progressive hormone refractory human prostate cancer. Am. J. Pathol. 144:735–746; 1994.
Russell, P. J.; Bennett, S.; Stricker, P. Growth factor involvement in progression of prostate cancer. Clin. Chem. 44:705–723; 1998.
Sehgal, I.; Thompson, T. C. Novel regulation of type IV collagenase (matrix metalloproteinase-9 and-2) activities by transforming growth factor-beta1 in human prostate cancer cell lines. Mol. Biol. Cell 10:407–416; 1999.
Skowronski, R. J.; Peehl, D. M.; Feldman, D. Vitamin D and prostate cancer: 1,25 dihydroxyvitamin D3 receptors and actions in human prostate cancer cell lines. Endocrinology 132:1952–1960; 1993.
Stearns, M.; Stearns, M. E. Evidence for increased activated metalloproteinase 2 (MMP-2a) expression associated with human prostate cancer progression. Oncol. Res. 8:69–75; 1996.
Story, M. T.; Hopp, K. A.; Molter, M. Expression of transforming growth factor beta 1 (TGF beta 1),-beta 2, and-beta 3 by cultured human prostate cells. J. Cell Physiol. 169:97–107; 1996.
Trapman, J.; Brinkmann, A. O. The androgen receptor in prostate cancer. Pathol. Res. Pract. 192:752–760; 1996.
Tuxhorn, J. A.; Ayala, G. E.; Rowley, D. R. Reactive stroma in prostate cancer progression. J. Urol. 166:2472–2483; 2001.
van Bokhoven, A.; Varella-Garcia, M.; Korch, C., et al. Molecular characterization of human prostate carcinoma cell lines. Prostate 57:205–225; 2003.
Walters, M. R.; Hunziker, W.; Konami, D.; Norman, A. W. Factors affecting the distribution and stability of unoccupied 1,25-dihydroxyvitamin D3 receptors. J. Recept. Res. 2:331–346; 1981–1982.
Walters, S. N.; Reinhardt, T. A.; Dominick, M. A.; Horst, R. L.; Littledike, E. T. Intracellular location of unoccupied 1,25-dihydroxyvitamin D receptors: a nuclear-cytoplasmic equilibrium. Arch. Biochem. Biophys. 246:366–373; 1986.
Webber, M. M.; Trakul, N.; Thraves, P. S., et al. A human prostatic stromal myofibroblast cell line WPMY-1: a model for stromal-epithelial interactions in prostatic neoplasia. Carcinogenesis 20:1185–1192; 1999.
Wilson, M. J. Proteases in prostate development, function, and pathology. Microsc. Res. Tech. 30:305–318; 1995.
Wilson, M. J.; Sellers, R. G.; Wiehr, C.; Melamud, O.; Pei, D.; Peehl, D. M. Expression of matrix metalloproteinase-2 and-9 and their inhibitors, tissue inhibitor of metalloproteinase-1 and-2, in primary cultures of human prostatic stromal and epithelial cells. J. Cell Physiol. 191:208–216; 2002.
Wong, Y. C.; Wang, Y. Z. Growth factors and epithelial-stromal interactions in prostate cancer development. Int. Rev. Cytol. 199:65–116; 2000.
Yu, H.; Rohan, T. Role of the insulin-like growth factor family in cancer development and progression. J. Natl. Cancer Inst. 92:1472–1489; 2000.
Author information
Authors and Affiliations
Corresponding author
Additional information
The content of this publication does not necessarily reflect the view or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. Government.
Rights and permissions
About this article
Cite this article
Diaw, L., Roth, M., Schwinn, D.A. et al. Characteristics of a human prostate stromal cell line related to its use in a stromal-epithelial coculture model for the study of cancer chemoprevention. In Vitro Cell.Dev.Biol.-Animal 41, 142–148 (2005). https://doi.org/10.1290/0412079.1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1290/0412079.1