Abstract
This study tests the hypothesis that association complexes formed between enoxaparin and cetyltrimethylammonium bromide (CTAB) augment permeation across the gastrointestinal mucosa due to improved encapsulation of this hydrophilic macromolecule within biocompatible poly (lactide-co-glycolide, PLGA RG 503) nanoparticles. When compared with free enoxaparin, association with CTAB increased drug encapsulation efficiency within PLGA nanoparticles from 40.3 ± 3.4 to 99.1 ± 1.0%. Drug release from enoxaparin/CTAB PLGA nanoparticles was assessed in HBSS, pH 7.4 and FASSIFV2, pH 6.5, suggesting effective protection of PLGA-encapsulated enoxaparin from unfavorable intestinal conditions. The stability of the enoxaparin/CTAB ion pair complex was pH-dependent, resulting in more rapid dissociation under simulated plasma conditions (i.e., pH 7.4) than in the presence of a mild acidic gastrointestinal environment (i.e., pH 6.5). The intestinal flux of enoxaparin complexes across in vitro Caco-2 cell monolayers was greater when encapsulated within PLGA nanoparticles. Limited changes in transepithelial transport of PLGA-encapsulated enoxaparin complexes in the presence of increasing CTAB concentrations suggest a significant contribution of size-dependent passive diffusion as the predominant transport mechanism facilitating intestinal absorption.

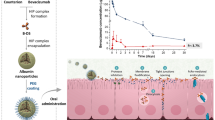
Graphical abstract








Similar content being viewed by others
References
Caputo HE, Straub JE, Grinstaff MW. Design, synthesis, and biomedical applications of synthetic sulphated polysaccharides. Chem Soc Rev. 2019;48(8):2338–65.
Zhou J, Fang R, Yan Q, Li C, Zhou Y, Nur AA, Liu T, Wang W. Low-molecular-weight heparin followed by rivaroxaban or not for the prevention of deep venous thromboembolism after total knee arthroplasty. Blood Coagul Fibrinolysis. 2019;30(1):29–33.
Dong W, Wang X, Liu C, Zhang X, Zhang X, Chen X, Kou Y, Mao S. Chitosan based polymer-lipid hybrid nanoparticles for oral delivery of enoxaparin. Int J Pharm. 2018;547(1–2):499–505.
Hayes PY, Ross BP, Thomas BG, Toth I. Polycationic lipophilic-core dendrons as penetration enhancers for the oral administration of low molecular weight heparin. Bioorg Med Chem. 2006;14(1):143–52.
Twarog C, Fattah S, Heade J, Maher S, Fattal E, Brayden DJ. Intestinal permeation enhancers for oral delivery of macromolecules: a comparison between salcaprozate sodium (SNAC) and sodium caprate (C10). Pharmaceutics. 2019;11(2):78.
He H, Lu Y, Qi J, Zhu Q, Chen Z, Wu W. Adapting liposomes for oral drug delivery. Acta Pharm Sin B. 2019;9(1):36–48.
Maher S, Brayden DJ, Casettari L, Illum L. Application of permeation enhancers in oral delivery of macromolecules: an update. Pharmaceutics. 2019;11(1):41.
Bernkop-Schnürch A. The use of inhibitory agents to overcome the enzymatic barrier to perorally administered therapeutic peptides and proteins. J Control Release. 1998;52(1–2):1–16.
Colzani B, Pandolfi L, Hoti A, Iovene PA, Natalello A, Avvakumova S, Colombo M, Prosperi D. Investigation of antitumor activities of trastuzumab delivered by PLGA nanoparticles. Int J Nanomedicine. 2018;13:957.
Pereverzeva E, Treschalin I, Treschalin M, Arantseva D, Ermolenko Y, Kumskova N, Maksimenko O, Balabanyan V, Kreuter J, Gelperina S. Toxicological study of doxorubicin-loaded PLGA nanoparticles for the treatment of glioblastoma. Int J Pharm. 2019;554:161–78.
Sah E, Sah H. Recent trends in preparation of poly (lactide-co-glycolide) nanoparticles by mixing polymeric organic solution with antisolvent. J Nanomater. 2015;2015:61.
Zaichik S, Steinbring C, Caliskan C, Bernkop-Schnürch A. Development and in vitro evaluation of a self-emulsifying drug delivery system (SEDDS) for oral vancomycin administration. Int J Pharm. 2019;554:125–33.
Phan TNQ, Le-Vinh B, Efiana NA, Bernkop-Schnürch A. Oral self-emulsifying delivery systems for systemic administration of therapeutic proteins: science fiction? Journal of Drug Targeting. 2019;27(9):1017–24.
Lozoya-Agullo I, Planelles M, Merino-Sanjuán M, Bermejo M, Sarmento B, González-Álvarez I, González-Álvarez M. Ion-pair approach coupled with nanoparticle formation to increase bioavailability of a low permeability charged drug. Int J Pharm. 2019;557:36–42.
Nazir I, Asim MH, Dizdarević A, Bernkop-Schnürch A. Self-emulsifying drug delivery systems: impact of stability of hydrophobic ion pairs on drug release. Int J Pharm. 2019;561:197–205.
Eleraky NE, Mohamed DF, Attia MA, Pauletti GM. Hydrophobic ion-pairing of low molecular weight heparin with cetyltrimethylammonium bromide. Int J Pharm Sci Res. 2015;6:558–65.
Cadène M, Boudier C, de Marcillac GD, Bieth JG. Influence of low molecular mass heparin on the kinetics of neutrophil elastase inhibition by mucus proteinase inhibitor (*). J Biol Chem. 1995;270(22):13204–9.
Fessi H, Puisieux F, Devissaguet JP, Ammoury N, Benita S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int J Pharm. 1989;55(1):R1–4.
D’souza SS, DeLuca PP. Methods to assess in vitro drug release from injectable polymeric particulate systems. Pharm Res. 2006;23(3):460–74.
Pauletti GM, Gangwar S, Okumu FW, Siahaan TJ, Stella VJ, Borchardt RT. Esterase-sensitive cyclic prodrugs of peptides: evaluation of an acyloxyalkoxy promoiety in a model hexapeptide. Pharm Res. 1996;13(11):1615–23.
Souza VMD, Shertzer HG, Menon AG, Pauletti GM. High glucose concentration in isotonic media alters Caco-2 cell permeability. AAPS PharmSci. 2015;5(3):17.
Kotzé AR, Lueβen HL, de Leeuw BJ, de Boer BG, Verhoef JC, Junginger HE. N-trimethyl chitosan chloride as a potential absorption enhancer across mucosal surfaces: in vitro evaluation in intestinal epithelial cells (Caco-2). Pharm Res. 1997;14(9):1197–202.
Haas S. The role of low molecular weight heparins for venous thromboembolism prevention in medical patients—what is new in 2019? Hämostaseologie. 2019;39 (01):062–6.
Zupančič O, Grieβinger JA, Rohrer J, de Sousa IP, Danninger L, Partenhauser A, Sündermann NE, Laffleur F, Bernkop-Schnürch A. Development, in vitro and in vivo evaluation of a self-emulsifying drug delivery system (SEDDS) for oral enoxaparin administration. Eur J Pharm Biopharm. 2016;109:113–21.
Holmkvist AD, Friberg A, Nilsson UJ, Schouenborg J. Hydrophobic ion pairing of a minocycline/Ca2+/AOT complex for preparation of drug-loaded PLGA nanoparticles with improved sustained release. Int J Pharm. 2016;499(1–2):351–7.
Banerjee A, Qi J, Gogoi R, Wong J, Mitragotri S. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J Control Release. 2016;238:176–85.
Caldorera-Moore M, Guimard N, Shi L, Roy K. Designer nanoparticles: incorporating size, shape and triggered release into nanoscale drug carriers. Expert Opinion on Drug Delivery. 2010;7(4):479–95.
Sun S, Liang N, Kawashima Y, Xia D, Cui F. Hydrophobic ion pairing of an insulin-sodium deoxycholate complex for oral delivery of insulin. Int J Nanomedicine. 2011;6:3049.
Kalaria D, Sharma G, Beniwal V, Kumar MR. Design of biodegradable nanoparticles for oral delivery of doxorubicin: in vivo pharmacokinetics and toxicity studies in rats. Pharm Res. 2009;26(3):492–501.
Jain AK, Swarnakar NK, Godugu C, Singh RP, Jain S. The effect of the oral administration of polymeric nanoparticles on the efficacy and toxicity of tamoxifen. Biomaterials. 2011;32(2):503–15.
Fredenberg S, Wahlgren M, Reslow M, Axelsson A. The mechanisms of drug release in poly (lactic-co-glycolic acid)-based drug delivery systems—a review. Int J Pharm. 2011;415(1):34–52.
Wang J, Wang BM, Schwendeman SP. Characterization of the initial burst release of a model peptide from poly (D, L-lactide-co-glycolide) microspheres. J Control Release. 2002;82(2):289–307.
Jiao Y, Ubrich N, Marchand-Arvier M, Vigneron C, Hoffman M, Lecompte T, Maincent P. In vitro and in vivo evaluation of oral heparin–loaded polymeric nanoparticles in rabbits. Circulation. 2002;105(2):230–5.
Makino K, Mogi T, Ohtake N, Yoshida M, Ando S, Nakajima T, Ohshima H. Pulsatile drug release from poly (lactide-co-glycolide) microspheres: how does the composition of the polymer matrices affect the time interval between the initial burst and the pulsatile release of drugs? Colloids Surf B: Biointerfaces. 2000;19(2):173–9.
Lewis R. Hawley’s condensed chemical dictionary. 15 e éd. A John Wiley & Sons. Inc, New York. 2007.
Sang Yoo H, Gwan PT. Biodegradable nanoparticles containing protein-fatty acid complexes for oral delivery of salmon calcitonin. J Pharm Sci. 2004;93(2):488–95.
Motlekar NA, Srivenugopal KS, Wachtel MS, Youan BBC. Modulation of gastrointestinal permeability of low-molecular-weight heparin by L-arginine: in-vivo and in-vitro evaluation. J Pharm Pharmacol. 2006;58(5):591–8.
Artursson P. Epithelial transport of drugs in cell culture. I: a model for studying the passive diffusion of drugs over intestinal absorbtive (Caco-2) cells. J Pharm Sci. 1990;79(6):476–82.
He C, Hu Y, Yin L, Tang C, Yin C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31(13):3657–66.
Acknowledgments
This research was supported in part by a predoctoral fellowship from the Egyptian Ministry of Higher Education awarded to Nermin E. Eleraky.
Author information
Authors and Affiliations
Contributions
Nermin E. Eleraky executed preparation and characterization of enoxaparin/CTAB complexes and enoxaparin/CTAB loaded PLGA nanoparticles, in vitro release of enoxaparin, enoxaparin/CTAB complex stability study using ITC, Caco-2 cell permeation study and manuscript preparation. Nitin K. Swarnakar helped with Caco-2 cell permeation studies. Dina F. Mohamed and Mohamed A. Attia participated in the experimental design and manuscript review. Giovanni M. Pauletti collaborated with experimental designs and aid during manuscript preparation. All authors read and confirmed the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
On behalf of all the authors, Nermin E. Eleraky, Ph.D. declares that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Eleraky, N.E., Swarnakar, N.K., Mohamed, D.F. et al. Permeation-Enhancing Nanoparticle Formulation to Enable Oral Absorption of Enoxaparin. AAPS PharmSciTech 21, 88 (2020). https://doi.org/10.1208/s12249-020-1618-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-1618-2