Abstract
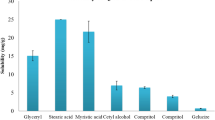
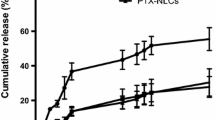
The present studies describe quality-by-design-based design and characterization of cationic self-nanoemulsifying formulations of paclitaxel for improving its biopharmaceutical attributes. Solubility and phase titration experiments were designed to select the lipidic and emulsifying excipients. Two different types of lipidic nanoformulations were developed using medium-chain triglycerides (MCTs) and long-chain triglycerides (LCTs). The nanoformulations were optimized by mixture designs and subjected to evaluation for globule size, zeta potential, drug release, and intestinal permeability. Following apt mathematical modeling, the optimum nanoformulation was earmarked using numerical optimization. Further, cationic formulations were developed for both LCT- and MCT-containing formulations and subjected to performance evaluation. The optimized formulations were extensively evaluated, where an in vitro drug release study indicated 2.7-fold improvement in dissolution rate from optimized cationic nanoformulations over powder pure drug. Ex vivo and in situ evaluation performed on Wistar rats exhibited nearly six- to eightfold enhancement in permeation and absorption parameters of the drug for the optimized cationic nanoformulation as compared to the pure paclitaxel. Pharmacokinetic studies indicated nearly 13.4-fold improvement in AUC and Cmax, along with 1.8-fold reduction in Tmax of the drug from cationic nanoformulations as compared to the pure drug suspension. Moreover, nanoformulation containing long-chain lipids exhibited superior performance (1.18-fold improvement in drug absorption) over medium-chain lipids. Cytotoxicity evaluation of cationic nanoformulations on MCF-7 cells revealed significant reduction in growth vis-à-vis the pure drug. Overall, the current paper reports successful systematic development of paclitaxel-loaded cationic self-nanoemulsifying systems with distinctly improved biopharmaceutical performance.









Similar content being viewed by others
References
Beg S, Swain S, Rizwan M, Irfanuddin M, Malini DS. Bioavailability enhancement strategies: basics, formulation approaches and regulatory considerations. Curr Drug Deliv. 2011;8:691–702.
Goldberg M, Gomez-Orellana I. Challenges for the oral delivery of macromolecules. Nat Rev Drug Discov. 2003;2:289–95.
Gomez-Orellana I. Strategies to improve oral drug bioavailability. Expert Opin Drug Deliv. 2005;2:419–33.
Kingston DG, Snyder JP. The quest for a simple bioactive analog of paclitaxel as a potential anticancer agent. Acc Chem Res. 2014;47:2682–91.
Chandra S. Endophytic fungi: novel sources of anticancer lead molecules. Appl Microbiol Biotechnol. 2012;95:47–59.
Stage TB, Bergmann TK, Kroetz DL. Clinical pharmacokinetics of paclitaxel monotherapy: an updated literature review. Clin Pharmacokinet. 2018;57:7–19.
Kearns CM, Gianni L, Egorin MJ. Paclitaxel pharmacokinetics and pharmacodynamics. Semin Oncol. 1995;22:16–23.
Sparreboom A, van Asperen J, Mayer U, Schinkel AH, Smit JW, Meijer DKF, et al. Limited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestine. Proc Natl Acad Sci U S A. 1997;94:2031–5.
Neslihan Gursoy R, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58:173–82.
Porter CJH, Trevaskis NL, Charman WN. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov. 2007;6:231–48.
Singh B, Bandyopadhyay S, Beg S, Katare OP. Handling poorly bioavailable drugs using nanoemulsifying drug delivery systems. Pharm Rev. 2011:91–8.
Singh B, Bandopadhyay S, Kapil R, Singh R, Katare O. Self-emulsifying drug delivery systems (SEDDS): formulation development, characterization, and applications. Crit Rev Ther Drug Carrier Syst. 2009;26:427–521.
Date AA, Desai N, Dixit R, Nagarsenker M. Self-nanoemulsifying drug delivery systems: formulation insights, applications and advances. Nanomedicine (Lond). 2010;5:1595–616.
Singh B, Beg S, Khurana RK, Sandhu PJS, Kaur R, Katare OP. Recent advances in self-emulsifying drug delivery systems (SEDDS). Crit Rev Ther Drug Carrier Syst. 2014;31:121–85.
O'Driscoll CM. Lipid-based formulations for intestinal lymphatic delivery. Eur J Pharm Sci. 2002;15:405–15.
Lo JT, Chen BH, Lee TM, Han J, Li JL. Self-emulsifying O/W formulations of paclitaxel prepared from mixed nonionic surfactants. J Pharm Sci. 2010;99:2320–32.
Sandhu PS, Beg S, Mehta F, Singh B, Trivedi P. Novel dietary lipid-based self-nanoemulsifying drug delivery systems of paclitaxel with p-gp inhibitor: implications on cytotoxicity and biopharmaceutical performance. Expert Opin Drug Deliv. 2015;12:1809–22.
Patel K, Patil A, Mehta M, Gota V, Vavia P. Medium chain triglyceride (MCT) rich, paclitaxel loaded self nanoemulsifying preconcentrate (PSNP): a safe and efficacious alternative to Taxol. J Biomed Nanotechnol. 2013;9:1996–2006.
Zhang XN, Tang LH, Gong JH, Yan XY, Zhang Q. An alternative paclitaxel self-emulsifying microemulsion formulation: preparation, pharmacokinetic profile, and hypersensitivity evaluation. PDA J Pharm Sci Technol. 2006;60:89–94.
Veltkamp SA, Thijssen B, Garrigue JS, Lambert G, Lallemand F, Binlich F, et al. A novel self-microemulsifying formulation of paclitaxel for oral administration to patients with advanced cancer. Br J Cancer. 2006;95:729–34.
Lionberger RA, Lee SL, Lee L, Raw A, Yu LX. Quality by design: concepts for ANDAs. AAPS J. 2008;10:268–76.
Yu LX, Amidon G, Khan MA, Hoag SW, Polli J, Raju GK, et al. Understanding pharmaceutical quality by design. AAPS J. 2014;16:771–83.
Beg S, Akhter S, Rahman M, Rahman Z. Perspectives of quality by design approach in nanomedicines development. Curr Nanomed. 2017;7:191–7.
Singh B, Kapil R, Nandi M, Ahuja N. Developing oral drug delivery systems using formulation by design: vital precepts, retrospect and prospects. Expert Opin Drug Deliv. 2011;8:1341–60.
Singh B, Kumar R, Ahuja N. Optimizing drug delivery systems using systematic “design of experiments.” Part I: fundamental aspects. Crit Rev Ther Drug Carrier Syst. 2005;22:27–105.
Singh B, Beg S, Raza K. Developing “optimized” drug products employing “designed” experiments. Chem Ind Digest. 2013;23:70–6.
Jain A, Kaur R, Beg S, Kushwah V, Jain S, Singh B. Novel cationic supersaturable nanomicellar systems of raloxifene hydrochloride with enhanced biopharmaceutical attributes. Drug Deliv Transl Res. 2018;8:670–92.
Beg S, Katare OP, Singh B. Formulation by design approach for development of ultrafine self-nanoemulsifying systems of rosuvastatin calcium containing long-chain lipophiles for hyperlipidemia management. Colloids Surf B Biointerfaces. 2017;159:869–79.
Khurana RK, Beg S, Burrow AJ, Vashishta RK, Katare OP, Kaur S, et al. Enhancing biopharmaceutical performance of an anticancer drug by long chain PUFA based self-nanoemulsifying lipidic nanomicellar systems. Eur J Pharm Biopharm. 2017;121:42–60.
Sandhu PS, Kumar R, Beg S, Jain S, Kushwah V, Katare OP, et al. Natural lipids enriched self-nano-emulsifying systems for effective co-delivery of tamoxifen and naringenin: systematic approach for improved breast cancer therapeutics. Nanomedicine. 2017;13:1703–13.
Tripathi CB, Beg S, Kaur R, Shukla G, Bandopadhyay S, Singh B. Systematic development of optimized SNEDDS of artemether with improved biopharmaceutical and antimalarial potential. Drug Deliv. 2016;23:3209–23.
Beg S, Sandhu PS, Batra RS, Khurana RK, Singh B. QbD-based systematic development of novel optimized solid self-nanoemulsifying drug delivery systems (SNEDDS) of lovastatin with enhanced biopharmaceutical performance. Drug Deliv. 2014;22:765–84.
Beg S, Jena SS, Patra Ch N, Rizwan M, Swain S, Sruti J, et al. Development of solid self-nanoemulsifying granules (SSNEGs) of ondansetron hydrochloride with enhanced bioavailability potential. Colloids Surf B Biointerfaces. 2013;101:414–23.
Beg S, Sharma G, Thanki K, Jain S, Katare OP, Singh B. Positively charged self-nanoemulsifying oily formulations of olmesartan medoxomil: systematic development, in vitro, ex vivo and in vivo evaluation. Int J Pharm. 2015;493:466–82.
Beg S, Swain S, Singh HP, Patra Ch N, Rao ME. Development, optimization, and characterization of solid self-nanoemulsifying drug delivery systems of valsartan using porous carriers. AAPS PharmSciTech. 2012;13:1416–27.
Beg S, Jena SS, Patra Ch N, Rizwan M, Swain S, Sruti J, et al. Development of solid self-nanoemulsifying granules (SSNEGs) of ondansetron hydrochloride with enhanced bioavailability potential. Colloids Surf B Biointerfaces. 2013;101:414–23.
Lee SH, Yoo SD, Lee KH. Rapid and sensitive determination of paclitaxel in mouse plasma by high-performance liquid chromatography. J Chromatogr B. 1999;724:357–63.
Bandyopadhyay S, Beg S, Katare OP, Sharma G, Singh B. QbD-oriented development of self-nanoemulsifying drug delivery systems (SNEDDS) of valsartan with improved biopharmaceutical performance. Curr Drug Deliv. 2015;12:544–63.
Bandyopadhyay S, Katare OP, Singh B. Optimized self nano-emulsifying systems of ezetimibe with enhanced bioavailability potential using long chain and medium chain triglycerides. Colloids Surf B Biointerfaces. 2012;100:50–61.
Daniel WW. Biostatistics: a foundation for analysis in the health sciences. 5th ed. New York: Wiley & Sons; 1991.
Singh B, Kaur A, Dhiman S, Garg B, Khurana RK, Beg S. QbD-enabled development of novel stimuli-responsive gastroretentive systems of acyclovir for improved patient compliance and biopharmaceutical performance. AAPS PharmSciTech. 2016;17:454–65.
Shah NH, Carvajal MT, Patel CI, Infeld MH, Malick AW. Self-emulsifying drug delivery systems (SEDDS) with polyglycolyzed glycerides for improving in vitro dissolution and oral absorption of lipophilic drugs. Int J Pharm. 1994;106:15–23.
Porter CJH, Trevaskis NL, Charman WN. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov. 2007;6:231–48.
Kang BK, Chon SK, Kim SH, Jeong SY, Kim MS, Cho SH, et al. Controlled release of paclitaxel from microemulsion containing PLGA and evaluation of anti-tumor activity in vitro and in vivo. Int J Pharm. 2004;286:147–56.
Xi J, Chang Q, Chan CK, Meng ZY, Wang GN, Sun JB, et al. Formulation development and bioavailability evaluation of a self-nanoemulsified drug delivery system of oleanolic acid. AAPS PharmSciTech. 2009;10:172–82.
Cuine JF, Charman WN, Pouton CW, Edwards GA, Porter CJ. Increasing the proportional content of surfactant (Cremophor EL) relative to lipid in self-emulsifying lipid-based formulations of danazol reduces oral bioavailability in beagle dogs. Pharm Res. 2007;24:748–57.
Grove M, Mullertz A, Nielsen JL, Pedersen GP. Bioavailability of seocalcitol II: development and characterisation of self-microemulsifying drug delivery systems (SMEDDS) for oral administration containing medium and long chain triglycerides. Eur J Pharm Sci. 2006;28:233–42.
Deckelbaum RJ, Hamilton JA, Moser A, Bengtsson-Olivecrona G, Butbul E, Carpentier YA, et al. Medium-chain versus long-chain triacylglycerol emulsion hydrolysis by lipoprotein lipase and hepatic lipase: implications for the mechanisms of lipase action. Biochemistry. 1990;29:1136–42.
Mullertz A, Ogbonna A, Ren S, Rades T. New perspectives on lipid and surfactant based drug delivery systems for oral delivery of poorly soluble drugs. J Pharm Pharmacol. 2010;62:1622–36.
Date AA, Nagarsenker MS. Design and evaluation of self-nanoemulsifying drug delivery systems (SNEDDS) for cefpodoxime proxetil. Int J Pharm. 2007;329:166–72.
Taha EI, Al-Saidan S, Samy AM, Khan MA. Preparation and in vitro characterization of self-nanoemulsified drug delivery system (SNEDDS) of all-trans-retinol acetate. Int J Pharm. 2004;285:109–19.
Beg S, Sandhu PS, Batra RS, Khurana RK, Singh B. QbD-based systematic development of novel optimized solid self-nanoemulsifying drug delivery systems (SNEDDS) of lovastatin with enhanced biopharmaceutical performance. Drug Deliv. 2015;22:765–84.
Johnson DA, Amidon GL. Determination of intrinsic membrane transport parameters from perfused intestine experiments: a boundary layer approach to estimating the aqueous and unbiased membrane permeabilities. J Theor Biol. 1988;131:93–106.
Madan J, Chawla G, Arora V, Malik R, Bansal AK. Unbiased membrane permeability parameters for gabapentin using boundary layer approach. AAPS J. 2005;7:E224–30.
Oh DM, Curl RL, Amidon GL. Estimating the fraction dose absorbed from suspensions of poorly soluble compounds in humans: a mathematical model. Pharm Res. 1993;10:264–70.
Chen Y, Li G, Wu X, Chen Z, Hang J, Qin B, et al. Self-microemulsifying drug delivery system (SMEDDS) of vinpocetine: formulation development and in vivo assessment. Biol Pharm Bull. 2008;31:118–25.
Sun M, Zhai X, Xue K, Hu L, Yang X, Li G, et al. Intestinal absorption and intestinal lymphatic transport of sirolimus from self-microemulsifying drug delivery systems assessed using the single-pass intestinal perfusion (SPIP) technique and a chylomicron flow blocking approach: linear correlation with oral bioavailabilities in rats. Eur J Pharm Sci. 2011;43:132–40.
Flesch G, Muller P, Lloyd P. Absolute bioavailability and pharmacokinetics of valsartan, an angiotensin II receptor antagonist, in man. Eur J Clin Pharmacol. 1997;52:115–20.
Saydam M, Takka S. Bioavailability file: valsartan. FABAD. J Pharm Sci. 2007;32:185–96.
Zhao G, Duan J, Xie Y, Lin G, Luo H, Li G, et al. Effects of solid dispersion and self-emulsifying formulations on the solubility, dissolution, permeability and pharmacokinetics of isorhamnetin, quercetin and kaempferol in total flavones of Hippophae rhamnoides L. Drug Dev Ind Pharm. 2012;39:1037–45.
Thomas N, Holm R, Mullertz A, Rades T. In vitro and in vivo performance of novel supersaturated self-nanoemulsifying drug delivery systems (super-SNEDDS). J Control Release. 2012;160:25–32.
Acknowledgments
The corresponding author gratefully acknowledges the generosity of M/s Stat-Ease Inc., Minneapolis, USA, for providing software support along with felicitation with “Stat-Ease QbD Performance Award 2014” for his exemplary contribution in the QbD-based drug delivery research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Guest Editor: Sanyog Jain
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 876 kb)
Rights and permissions
About this article
Cite this article
Beg, S., Kaur, R., Khurana, R.K. et al. QbD-Based Development of Cationic Self-nanoemulsifying Drug Delivery Systems of Paclitaxel with Improved Biopharmaceutical Attributes. AAPS PharmSciTech 20, 118 (2019). https://doi.org/10.1208/s12249-019-1319-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1319-x