Abstract
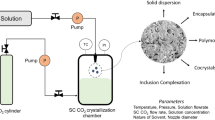
Tacrolimus is an immunosuppressant agent that suffers from poor and variable bioavailability. This can be related to limited solubility and dissolution. The main objective of this study is to use SFT to prepare solid dispersions of tacrolimus in order to enhance its dissolution. SFT was selected since it offers several advantages over conventional techniques such as efficiency and stability. Several solid dispersions of tacrolimus were prepared using SFT to enhance its dissolution. The selected polymers included soluplus, PVP, HPMC, and porous chitosan. TPGS was used as a surfactant additive with chitosan, HPMC, and PVP. Soluplus dispersions were used to study the effect of processing parameters (time, temperature, and pressure) on loading efficiency (LE) and dissolution of the preparation. Physicochemical characterization was performed using DSC, X-ray diffraction, FTIR analysis, SEM, and in vitro drug release. Stability testing was evaluated after 3 months for selected dispersions. Significant improvement for the release profile was achieved for the prepared dispersions. Better release achieved in the soluplus dispersions which reached maximum cumulative release equal to 98.76% after 24 h. Drug precipitated in its amorphous form in all prepared dispersions except those prepared from chitosan. All dispersions were physically stable except for PVP preparations that contained TPGS which started to re-crystallize after one month. Prepared dispersions were proved to be affected by supercritical processing parameters. In conclusion, SFT was successfully used to prepare dispersions of tacrolimus that exhibited higher dissolution than raw drug. Dissolution rate and stability are affected by the type of the polymer.







Similar content being viewed by others
References
Watts AB, Cline AM, Saad AR, Johnson SB, Peters JI, Williams RO. Characterization and pharmacokinetic analysis of tacrolimus dispersion for nebulization in a lung transplanted rodent model. Int J Pharm. 2010;384(1):46–52.
Park YJ, Ryu DS, Li DX, Quan QZ, Oh DH, Kim JO, et al. Physicochemical characterization of tacrolimus-loaded solid dispersion with sodium carboxylmethyl cellulose and sodium lauryl sulfate. Arch Pharm Res. 2009;32(6):893–8.
Shin SB, Cho HY, Kim DD, Choi HG, Lee YB. Preparation and evaluation of tacrolimus-loaded nanoparticles for lymphatic delivery. Eur J Pharm Biopharm. 2010;74(2):164–71.
Sinswat P, Overhoff KA, McConville JT, Johnston KP, Williams RO. Nebulization of nanoparticulate amorphous or crystalline tacrolimus–single-dose pharmacokinetics study in mice. Eur J Pharm Biopharm. 2008;69(3):1057–66.
Tamura S, Ohike A, Ibuki R, Amidon GL, Yamashita S. Tacrolimus is a class II low‐solubility high‐permeability drug: the effect of P‐glycoprotein efflux on regional permeability of tacrolimus in rats. J Pharm Sci. 2002;91(3):719–29.
Pandya P, Gattani S, Jain P, Khirwal L, Surana S. Co-solvent evaporation method for enhancement of solubility and dissolution rate of poorly aqueous soluble drug simvastatin: in vitro–in vivo evaluation. AAPS PharmSciTech. 2008;9(4):1247–52.
Shinde SS, Patil SS, Mevekari FI, Satpute AS. An approach for solubility enhancement: solid dispersion. Int J Adv Pharm Sci. 2010;1(3):299–308.
Sinha S, Ali M, Baboota S, Ahuja A, Kumar A, Ali J. Solid dispersion as an approach for bioavailability enhancement of poorly water-soluble drug ritonavir. AAPS PharmSciTech. 2010;11(2):518–27.
Sarode AL, Sandhu H, Shah N, Malick W, Zia H. Hot melt extrusion (HME) for amorphous solid dispersions: predictive tools for processing and impact of drug–polymer interactions on supersaturation. Eur J Pharm Sci. 2013;48(3):371–84.
Betageri GV, Makarla KR. Enhancement of dissolution of glyburide by solid dispersion and lyophilization techniques. Int J Pharm. 1995;126(1):155–60.
Seo A, Holm P, Kristensen HG, Schæfer T. The preparation of agglomerates containing solid dispersions of diazepam by melt agglomeration in a high shear mixer. Int J Pharm. 2003;259(1):161–71.
Jung JY, Yoo SD, Lee SH, Kim KH, Yoon DS, Lee KH. Enhanced solubility and dissolution rate of itraconazole by a solid dispersion technique. Int J Pharm. 1999;187(2):209–18.
Yang G, Zhao Y, Feng N, Zhang Y, Liu Y, Dang B. Improved dissolution and bioavailability of silymarin delivered by a solid dispersion prepared using supercritical fluids. Asian J Pharm Sci. 2015;10:194–202.
Kim MS, Kim JS, Park HJ, Cho WK, Cha KH, Hwang SJ. Enhanced bioavailability of sirolimus via preparation of solid dispersion nanoparticles using a supercritical antisolvent process. Int J Nanomedicine. 2011;6:2997–3009.
Singh MC, Sayyad AB, Sawant SD. Review on various techniques of solubility enhancement of poorly soluble drugs with special emphasis on solid dispersion. J Pharm Res. 2010;3(10):2494–501.
Das SK, Roy S, Kalimuthu Y, Khanam J, Nanda A. Solid dispersions: an approach to enhance the bioavailability of poorly water-soluble drugs. Int J Pharm Pharm Technol. 2012;1(1):37–46.
Sridhar I, Doshi A, Joshi B, Wankhede V, Doshi J. Solid dispersions: an approach to enhance solubility of poorly water soluble drug. J Sci Innov Res. 2013;2(3):685–94.
Nakamichi K, Yasuura H, Kukui H, Oka M, Izumi S, Andou T, et al. New preparation method of solid dispersion by twin screw extruder. Pharm Technol Jpn. 1996;12:715–29.
Obaidat RM, Tashtoush BM, Bayan MF, Al Bustami RT, Alnaief M. Drying using supercritical fluid technology as a potential method for preparation of chitosan aerogel microparticles. AAPS PharmSciTech. 2015;16(6):1235–44.
Berche B, Henkel M, Kenna R. Critical phenomena: 150 years since Cagniard de la Tour. J Phys Stud. 2009;13(3):3201–9.
Hannay JB, Hogarth J. On the solubility of solids in gases. Sci Am. 1880;9:3585–6.
Yasuji T, Takeuchi H, Kawashima Y. Particle design of poorly water-soluble drug substances using supercritical fluid technologies. Adv Drug Deliv Rev. 2008;60(3):388–98.
Pasquali I, Bettini R, Giordano F. Supercritical fluid technologies: an innovative approach for manipulating the solid-state of pharmaceuticals. Adv Drug Deliv Rev. 2008;60(3):399–410.
Lim RT, Ng WK, Tan RB. Amorphization of pharmaceutical compound by co-precipitation using supercritical anti-solvent (SAS) process (Part I). J Supercrit Fluids. 2010;53(1):179–84.
Prajapati D, Gohain M. Recent advances in the application of supercritical fluids for carbon–carbon bond formation in organic synthesis. Tetrahedron. 2004;60(4):815–33.
Alavi SH, Rizvi SS, Harriott P. Process dynamics of starch-based microcellular foams produced by supercritical fluid extrusion. I: model development. Food Res Int. 2003;36(4):309–19.
Gross J, Coronado PR, Hrubesh LW. Elastic properties of silica aerogels from a new rapid supercritical extraction process. J Non-Cryst Solids. 1998;225:282–6.
Park J, Cho W, Cha KH, Ahn J, Han K, Hwang SJ. Solubilization of the poorly water soluble drug, telmisartan, using supercritical anti-solvent (SAS) process. Int J Pharm. 2013;441(1):50–5.
Lim RT, Ng WK, Widjaja E, Tan RB. Comparison of the physical stability and physicochemical properties of amorphous indomethacin prepared by co-milling and supercritical anti-solvent co-precipitation. J Supercrit Fluids. 2013;79:186–201.
Edwards AD, Shekunov BY, Kordikowski A, Forbes RT, York P. Crystallization of pure anhydrous polymorphs of carbamazepine by solution enhanced dispersion with supercritical fluids (SEDS™). J Pharm Sci. 2001;90(8):1115–24.
Van den Mooter G, Augustijns P, Blaton N, Kinget R. Physico-chemical characterization of solid dispersions of temazepam with polyethylene glycol 6000 and PVP K30. Int J Pharm. 1998;164(1):67–80.
Sethia S, Squillante E. Solid dispersion of carbamazepine in PVP K30 by conventional solvent evaporation and supercritical methods. Int J Pharm. 2004;272(1):1–10.
Frizon F, de Oliveira EJ, Donaduzzi CM, Mitsui ML, Marchetti JM. Dissolution rate enhancement of loratadine in polyvinylpyrrolidone K-30 solid dispersions by solvent methods. Powder Technol. 2013;235:532–9.
Pham AT, Lee PI. Probing the mechanisms of drug release from hydroxypropylmethyl cellulose matrices. Pharm Res. 1994;11(10):1379–84.
Oh MJ, Shim JB, Yoo H, Lee GY, Jo H, Jeong SM, et al. The dissolution property of raloxifene HCl solid dispersion using hydroxypropyl methylcellulose. Macromol Res. 2012;20(8):835–41.
Nagy ZK, Balogh A, Vajna B, Farkas A, Patyi G, Kramarics Á, et al. Comparison of electrospun and extruded soluplus®‐based solid dosage forms of improved dissolution. J Pharm Sci. 2012;101(1):322–32.
Hardung H, Djuric D, Ali S. Combining HME & solubilization: Soluplus®—the solid solution. Drug Deliv Technol. 2010;10(3):20–7.
Portero A, Remunan-Lopez C, Vila-Jato JL. Effect of chitosan and chitosan glutamate enhancing the dissolution properties of the poorly water soluble drug nifedipine. Int J Pharm. 1998;175(1):75–84.
Fukuda M, Peppas NA, McGinity JW. Properties of sustained release hot-melt extruded tablets containing chitosan and xanthan gum. Int J Pharm. 2006;310(1):90–100.
Zhang Z, Tan S, Feng SS. Vitamin E TPGS as a molecular biomaterial for drug delivery. Biomaterials. 2012;33(19):4889–906.
Shin SC, Kim J. Physicochemical characterization of solid dispersion of furosemide with TPGS. Int J Pharm. 2003;251(1):79–84.
Obaidat R, Al-Jbour N, Al-Sou’d K, Sweidan K, Al-Remawi M, Badwan A. Some physico-chemical properties of low molecular weight chitosans and their relationship to conformation in aqueous solution. J Solut Chem. 2010;39(4):575–88.
Fule R, Meer T, Sav A, Amin P. Solubility and dissolution rate enhancement of lumefantrine using hot melt extrusion technology with physicochemical characterisation. J Pharm Invest. 2013;43(4):305–21.
Yamashita K, Nakate T, Okimoto K, Ohike A, Tokunaga Y, Ibuki R, et al. Establishment of new preparation method for solid dispersion formulation of tacrolimus. Int J Pharm. 2003;267(1):79–91.
Shi Q, Li J, Ding F. Development and validation of method for the determination of related substances of Tacrolimus in Tacrolimus capsules and degradation studies. Int J ChemTech Res. 2012;4:1543–52.
Shoaib MH, Tazeen J, Merchant HA, Yousuf RI. Evaluation of drug release kinetics from ibuprofen matrix tablets using HPMC. Pak J Pharm Sci. 2006;19(2):119–24.
Parize AL, Stulzer HK, Laranjeira MC, Brighente IM, Souza TC. Evaluation of chitosan microparticles containing curcumin and crosslinked with sodium tripolyphosphate produced by spray drying. Quim Nova. 2012;35(6):1127–32.
Riekes MK, Kuminek G, Rauber GS, de Campos CE, Bortoluzzi AJ, Stulzer HK. HPMC as a potential enhancer of nimodipine biopharmaceutical properties via ball-milled solid dispersions. Carbohydr Polym. 2014;99:474–82.
Goddeeris C, Willems T, Houthoofd K, Martens JA, Van den Mooter G. Dissolution enhancement of the anti-HIV drug UC 781 by formulation in a ternary solid dispersion with TPGS 1000 and Eudragit E100. Eur J Pharm Biopharm. 2008;70(3):861–8.
Patel PV, Panchal SS, Mehta TA. Improvement of dissolution rate of tacrolimus by solid dispersion technique. J Pharm Investig. 2013;43(1):45–53.
Silva SML, Braga CRC, Fook MVL, Raposo CMO, Carvalho LH, Canedo EL. Application of infrared spectroscopy to analysis of chitosan/clay nanocomposites. In: Theophanides T, editor. Infrared spectroscopy - materials science, engineering and technology. Croatia: InTech; 2012. p. 43–62.
Islam MM, Masum SM, Rahman MM, Molla MA, Shaikh AA, Roy SK. Preparation of chitosan from shrimp shell and investigation of its properties. Int J Basic Appl Sci. 2011;11(1):116–30.
Yu H, Xia D, Zhu Q, Zhu C, Chen D, Gan Y. Supersaturated polymeric micelles for oral cyclosporine A delivery. Eur J Pharm Biopharm. 2013;85(3):1325–36.
Joe JH, Lee WM, Park YJ, Joe KH, Oh DH, Seo YG, et al. Effect of the solid-dispersion method on the solubility and crystalline property of tacrolimus. Int J Pharm. 2010;395(1):161–6.
Yoshida T, Kurimoto I, Yoshihara K, Umejima H, Ito N, Watanabe S, et al. Aminoalkyl methacrylate copolymers for improving the solubility of tacrolimus. I: evaluation of solid dispersion formulations. Int J Pharm. 2012;428(1):18–24.
Zidan AS, Rahman Z, Sayeed V, Raw A, Yu L, Khan MA. Crystallinity evaluation of tacrolimus solid dispersions by chemometric analysis. Int J Pharm. 2012;423(2):341–50.
Ha JM, Kang SY, Park CW, Bin SA, Rhee YS, Seo JW, et al. Effect of poloxamer on physicochemical properties of tacrolimus solid dispersion improving water solubility and dissolution rate. J Pharm Investig. 2012;42(4):171–6.
Kittur FS, Prashanth KH, Sankar KU, Tharanathan RN. Characterization of chitin, chitosan and their carboxymethyl derivatives by differential scanning calorimetry. Carbohydr Polym. 2002;49(2):185–93.
Zhang CH, Zhao BX, Huang Y, Wang Y, Ke XY, Zhao BJ, et al. A novel domperidone hydrogel: preparation, characterization, pharmacokinetic, and pharmacodynamic properties. J Drug Deliv. 2011;2011:1–9.
Kiss D, Zelkó R, Novák C, Éhen Z. Application of DSC and NIRS to study the compatibility of metronidazole with different pharmaceutical excipients. J Therm Anal Calorim. 2006;84(2):447–51.
Gong K, Rehman IU, Darr JA. Characterization and drug release investigation of amorphous drug–hydroxypropyl methylcellulose composites made via supercritical carbon dioxide assisted impregnation. J Pharm Biomed Anal. 2008;48(4):1112–9.
Gong K, Darr JA, Rehman IU. Supercritical fluid assisted impregnation of indomethacin into chitosan thermosets for controlled release applications. Int J Pharm. 2006;315(1):93–8.
Zhang K, Yu H, Luo Q, Yang S, Lin X, Zhang Y, et al. Increased dissolution and oral absorption of itraconazole/Soluplus extrudate compared with itraconazole nanosuspension. Eur J Pharm Biopharm. 2013;85(3):1285–92.
Shamma RN, Basha M. Soluplus®: a novel polymeric solubilizer for optimization of Carvedilol solid dispersions: formulation design and effect of method of preparation. Powder Technol. 2013;237:406–14.
Thakral NK, Ray AR, Majumdar DK. Eudragit S-100 entrapped chitosan microspheres of valdecoxib for colon cancer. J Mater Sci Mater Med. 2010;21(9):2691–9.
Linn M, Collnot EM, Djuric D, Hempel K, Fabian E, Kolter K, et al. Soluplus® as an effective absorption enhancer of poorly soluble drugs in vitro and in vivo. Eur J Pharm Sci. 2012;45(3):336–43.
Mendiratta C, Kadam V, Pokharkar V. Lansoprazole solid dispersion using a novel amphiphillic polymer Soluplus®. J Chem Pharm Res. 2011;3:536–43.
Hughey JR, Keen JM, Miller DA, Kolter K, Langley N, McGinity JW. The use of inorganic salts to improve the dissolution characteristics of tablets containing Soluplus®-based solid dispersions. Eur J Pharm Sci. 2013;48(4):758–66.
Uzun İN, Sipahigil O, Dinçer S. Coprecipitation of Cefuroxime Axetil–PVP composite microparticles by batch supercritical antisolvent process. J Supercrit Fluids. 2011;55(3):1059–69.
Shavi GV, Kumar AR, Usha YN, Armugam K, Ranjan O, Ginjupalli K, et al. Enhanced dissolution and bioavailability of gliclazide using solid dispersion techniques. Int J Drug Deliv. 2010;2(1):49–57.
Ha ES, Baek IH, Cho W, Hwang SJ, Kim MS. Preparation and evaluation of solid dispersion of atorvastatin calcium with Soluplus® by spray drying technique. Chem Pharm Bull. 2014;62(6):545–51.
Gong K, Viboonkiat R, Rehman IU, Buckton G, Darr JA. Formation and characterization of porous indomethacin‐PVP coprecipitates prepared using solvent‐free supercritical fluid processing. J Pharm Sci. 2005;94(12):2583–90.
Braga ME, Costa VP, Pereira MJ, Fiadeiro PT, Gomes AP, Duarte CM, et al. Effects of operational conditions on the supercritical solvent impregnation of acetazolamide in Balafilcon A commercial contact lenses. Int J Pharm. 2011;420(2):231–43.
Kikic I. Polymer–supercritical fluid interactions. J Supercrit Fluids. 2009;47(3):458–65.
Durling NE, Catchpole OJ, Tallon SJ, Grey JB. Measurement and modelling of the ternary phase equilibria for high pressure carbon dioxide–ethanol–water mixtures. Fluid Phase Equilib. 2007;252(1):103–13.
Hussein Kh, Türk M, Wahl M. Preparation and evaluation of drug / ß-Cyclodextrin solid inclusion complexes by supercritical fluid technology. Proceedings of the 9th Meeting on SCF’s, Trieste, Italy, June 13–16, 2004.
Díez-Municio M, Montilla A, Herrero M, Olano A, Ibáñez E. Supercritical CO 2 impregnation of lactulose on chitosan: a comparison between scaffolds and microspheres form. J Supercrit Fluids. 2011;57(1):73–9.
Yañez F, Martikainen L, Braga ME, Alvarez-Lorenzo C, Concheiro A, Duarte CM, et al. Supercritical fluid-assisted preparation of imprinted contact lenses for drug delivery. Acta Biomater. 2011;7(3):1019–30.
Sambath L, Muthu AK, Kumar MA. Soluplus complexation influence the release of lovastatin from porous osmotic pump tablet. World J Pharm Pharm Sci. 2013;2(5):3506–21.
Khan AW, Kotta S, Ansari SH, Sharma RK, Ali J. Enhanced dissolution and bioavailability of grapefruit flavonoid Naringenin by solid dispersion utilizing fourth generation carrier. Drug Dev Ind Pharm. 2015;41(5):772–9.
Mitra A, Dey B. Chitosan microspheres in novel drug delivery systems. Ind J Pharm Sci. 2011;73(4):355–66.
Mi FL, Wong TB, Shyu SS, Chang SF. Chitosan microspheres: modification of polymeric chem‐physical properties of spray‐dried microspheres to control the release of antibiotic drug. J Appl Polym Sci. 1999;71(5):747–59.
Acknowledgments
This work was supported by the Jordan University of Science and Technology (Grant number 140/2013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Obaidat, R.M., Tashtoush, B.M., Awad, A.A. et al. Using Supercritical Fluid Technology (SFT) in Preparation of Tacrolimus Solid Dispersions. AAPS PharmSciTech 18, 481–493 (2017). https://doi.org/10.1208/s12249-016-0492-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0492-4