Abstract
In the last three decades, a lot of scientific research has been carried out in the field of Carbon nanomaterials all over the world due to their significant electronic, optical, mechanical, chemical and thermal properties. The zero, one, two and three dimensional Carbon nanomaterials (i.e. fullerenes, Carbon nanotubes, Graphene, Carbon quantum dots, Carbon Nanohorns, Nanodiamonds, Carbon Nanofibres and Carbon black) have exhibited such inherent features that can be easily exploited in the development of advanced technology for sensing applications. The employment of nanomaterials within sensors has paved new way and opportunities for the detection of analytes or target molecules. Carbon nanomaterials based electrochemical biosensors have reported biocompatibility, better sensitivity, better selectivity and lower limits of detection to detect a wide range of chemical to biological molecules. In this paper, a comprehensive review has been made to cover recent developments in the field of Carbon based nanomaterials as electrochemical biosensors. The characteristic features of a variety of nanomaterials like fullerenes, Carbon nanotubes, Graphene, Carbon quantum dots, Carbon Nanohorns, Carbon Nanodiamonds, Carbon Nanofibres, Carbon black etc. have been discussed along with their synthesis methods. The recent application of all these nanomaterials as electrochemical biosensors for the detection of various biomolecules have been highlighted; the future prospects and possibilities in this field have been outlined.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
The Carbon nanomaterials have laid down their historical footprints in the field of scientific research with the first investigations on fullerenes and related compounds during mid-eighties. Since then there has been a remarkable increase in the vast scientific research all over the world to innovate technology to study and develop this wonderful class of materials for a wide range of applications. Several new classes of nanomaterials have been investigated and reported since then with inherent intrinsic features for their viable application in the development of sensing and bio sensing devices. The Carbon has been used for the electrochemical electrodes due to its unique electrochemical properties i.e. a large potential window, low cost, a very small background current. The biocompatibility of Carbon nanomaterials has revolutionized the field of electrochemical detection of various analytes or targets. The electrochemical analysis has been used for the qualitative and quantitative determination of amount of electro active analytes. These methods have been highly accurate, reliable and cheap. The various characterization techniques have been employed for getting the electrochemical response like cyclic voltammetry (CV), Differential Pulse Voltammetry (DPV), Square Wave Voltammetry (SWV) or Pulsed Amperometry (PA).
In this paper, a comprehensive study has been made to highlight the characteristic features of a variety of nanomaterials along with the methods employed for their synthesis. The recent developments in the field of Carbon based nanomaterials for their use as electrochemical biosensors have been thoroughly discussed along with the future prospects and possibilities in this field.
Carbon Based Nanomaterials
The Carbon atoms, possessing a valency of four, have the ability to form single, double and triple covalent bonds among themselves or with other elements. Not only this, they have got the ability to form long chains of atoms, thus exhibiting the phenomenon of polymerization. The Carbon atoms do posses such an electronic structure and atomic size that makes them capable to exhibit distinct physical structures with distinct physical properties in spite of the same chemical composition. The Carbon atoms can undergo sp, sp2, sp3 hybridizations with a narrow band gap between their 2s and 2p electronic shells.1–3 The diamond with sp3 hybridization and graphite with sp2 hybridization are the two widely known allotropic forms of Carbon. The geometrical structure of the particles in nanomaterials is the basic criterion for their classification. The particles can have shapes of tubes, horns, spheres or ellipsoids. The tube or horn shaped particles are called as Carbon nanotubes (CNTs) or Carbon nanohorns (CNHs) respectively; the spherical or ellipsoidal nanoparticles are present in fullerenes.4–6 The CNMs find vast technical applications in micro and nanoelectronics, gas storage, production of conductive plastics, composites, paints, textiles, batteries with enhanced life times, biosensors, etc. attributed to their low toxic nature and large scale production for use.7,8 The fullerene, Carbon nanotubes (CNTs), Graphene, Carbon Nanodiamonds (CNDs) and Carbon dots (CDs) are the most significant allotropic modifications of the nanocarbon.9 The 0D nanodiamonds, 1D nanotubes, 2D Graphene nanosheets can act as a prototype for the nano composites.
Fullerenes
The allotropic modification of Carbon, known as fullerene, was discovered in 1985 by H. W. Kroto, R. F. Curl, and R. F. Smalley.10 It was the first nanomaterial to be successfully isolated. The characteristic feature of fullerenes is the formation of a number of atomic Cn clusters (n > 20) of carbon atoms on a spherical surface. The carbon atoms form covalent bonds with each other in the sp2 hybridization in fullerenes. They are most commonly present on the surface of the sphere at the vertices of pentagons and hexagons. C60 is the fullerene that has been extensively studied and investigated. It has highly symmetric spherical molecules consisting of 60 carbon atoms, present at the vertices of 20 hexagons and 12 pentagons or 60 Carbon atoms comprising of 12-five member rings and 20-six member rings.11 The diameter of fullerene is 0.7 nm.12 Fullerenes have been used in the medical field such as in cancer therapies, MRI and for gynecological malignancies.13–17
Synthesis
Fullerenes are basically 0D form of Graphitic carbon and can be visualized as an irregular sheet of Graphene being curled up into a sphere by adding pentagons at its structure. They come in various forms and sizes ranging from 30 to 300 Carbon atoms. They can be synthesized by employing methods such as electric arc-discharge, electron beam ablation and sputtering.18,19 Fullerenes are also present in the soot of combustion flames20–22 and can also be synthesized by using Graphitic electrodes.23,24 Fullerenes were firstly synthesized by evaporating Graphite electrodes in a Helium atmosphere.25,26 However, the practical use of fullerenes is limited due to their high synthesis cost and low yields of the methods currently available for their production.
Carbon nanotubes
One of the allotropic modifications of carbon, known as Carbon nanotubes (CNTs) were discovered in 1991 by the Japanese scientist S. Ijima.27 In CNTs, each carbon atom with 3 electrons forms trigonally coordinated  bonds to three carbon atoms by using sp2 hybridization.27–29 CNT is basically one layer of Graphene rolled in the form of a hollow tube seamlessly. The rolled Graphene sheets stacked in cylindrical/tubular structures with a diameter of several nanometers is the characteristic feature of carbon nanotubes. The CNTs can have variable length, diameter, the number of layers and chirality vectors (symmetry of the nulled Graphite sheet). Based on their structures, CNTs can be divided into two basic groups: single walled Carbon nanotubes (SWCNTs) and multi-walled Carbon nanotubes (MWCNTs).30,31 The SWCNTs have a diameter around 1–3 nm and a length of few micrometers whereas MWCNTs have a diameter of 5–25 nm and a length around 10 μm. However, recently the synthesis of CNTs with a length of 550 nm has been investigated and reported.32 The CNTs have excellent physical properties like rigidity, strength and elasticity as compared to other fibrous materials. They do posses high value of aspect ratio (length to diameter ratio) than other materials. The high aspect ratios of CNTs may vary from 102 to 107. The larger aspect ratio comes out for SWCNTs than MWCNTs as a consequence of their smaller diameter. Not only this, they do posses high thermal and electrical conductivities in comparison to other conductive materials. The strength of CNTs is 10–100 times larger than the strong steel at a fraction of steel weight.33
bonds to three carbon atoms by using sp2 hybridization.27–29 CNT is basically one layer of Graphene rolled in the form of a hollow tube seamlessly. The rolled Graphene sheets stacked in cylindrical/tubular structures with a diameter of several nanometers is the characteristic feature of carbon nanotubes. The CNTs can have variable length, diameter, the number of layers and chirality vectors (symmetry of the nulled Graphite sheet). Based on their structures, CNTs can be divided into two basic groups: single walled Carbon nanotubes (SWCNTs) and multi-walled Carbon nanotubes (MWCNTs).30,31 The SWCNTs have a diameter around 1–3 nm and a length of few micrometers whereas MWCNTs have a diameter of 5–25 nm and a length around 10 μm. However, recently the synthesis of CNTs with a length of 550 nm has been investigated and reported.32 The CNTs have excellent physical properties like rigidity, strength and elasticity as compared to other fibrous materials. They do posses high value of aspect ratio (length to diameter ratio) than other materials. The high aspect ratios of CNTs may vary from 102 to 107. The larger aspect ratio comes out for SWCNTs than MWCNTs as a consequence of their smaller diameter. Not only this, they do posses high thermal and electrical conductivities in comparison to other conductive materials. The strength of CNTs is 10–100 times larger than the strong steel at a fraction of steel weight.33
The one layer of Graphene in CNTs can be rolled in different ways. Based on the rolling of Graphene sheets, the CNTs are classified as zigzag, armchair, chiral, depending on the number of unit vectors in the crystal lattice of Graphene along two directions in honey comb structure. The chirality has a significant effect on the properties of CNTs. The electrical properties of SWCNTs are a function of their chirality or hexagon orientation with respect to the tube axis. The chirality decides whether a particular CNT is metallic or semiconducting in nature.34 The electrochemical properties of SWCNTs depend upon the roll-up vectors (n, m). If the roll-up vectors n-m = 3q where q is any integer/zero, the SWCNTs are metallic. If n-m = 3q, the SWCNTs are semi conductive in nature.35,36 If n = m, the nanotubes are known as armchair. If m = 0, they are known as zigzag, otherwise they are known as chiral.
Also, the SWCNTs can exhibit electrical conductivity or semi conductive properties that depend upon the diameter of the tubes.37–41 The armchair SWCNTs have electrical conductivity more than that of copper whereas zigzag and chiral SWCNTs do display semi conductive properties for their use in sensor fabrication.40,42 The MWCNTs are composed of multiple Carbon layers with inconsistent chirality and can display extraordinary mechanical attributes instead of exceptional electrical characteristics. These nanomaterials do posses such characteristic feature that makes them potential candidates for use in technological fields. The CNTs have been used as an electrode in electrochemical reactions due to their significant electron transfer capabilities.43 They can be used in electrochemical sensors as they do have the ability to make electron transfer possible in chemical reactions at the electrode interface.44–47 The CNTs find immense applications in the field of nano-electro-mechanical systems.48–50 Table I shows the values of significant physical, electronic and mechanical characteristic features of CNTs.
Table I. Significant physical, electronic and mechanical characteristic features of CNTs.51
| Specific surface area | 200–900 m2J−1 |
| Specific gravity | 0.8–2 g−1cm−2 |
| Electrical conductivity | 2 × 10−2−0.25 Scm−1 |
| Thermal conductivity | 6600 Wm−1K−1 |
| Elastic Modulus | >1 TPA |
| Tensile strength | >100 GPa |
Synthesis
The CNTs have been synthesized by using Carbon arc discharge, Chemical Vapour Deposition (CVD) and laser ablation methods.52–54 The Carbon arc discharge with a suitable catalyst was firstly used to synthesize SWCNTs or MWCNTs with a high yield and better control over the size of the synthesized nanotubes.27,55,56 The CVD method has resulted in the production of CNTs with smaller diameters and lower yield but finer quality.57 The Laser ablation method gives a lower yield and much smaller diameter but much finer quality.58,59 The metallic and semi conductive CNTs can be synthesized through selective functionalization,60 selective destruction by electrical heating61 or separation by density gradient ultra centrifugation.62 The CVD has been used to produce high quality SWCNTs and MWCNTs in vertically aligned array by using transition metal nanoparticle catalysts.63,64 They have been synthesized on a very large scale by using arc discharge and CVD methods (Co-Mo Catalysts). The CVD method needs simple equipment and mild temperature and pressure conditions and is more suitable for the large scale production of CNTs than the other two methods.65
The metallic and quasi crystalline substrates have been used to synthesize vertically aligned arrays of CNTs.66,67 The synthesis of CNTs has been reported by pyrolysing metal carbonyls in the presence of other hydrocarbons.68,69 The transition metals present in Graphite electrodes have produced CNTs with more product output and reproducibility.70 The transition metal catalysts along with CVD method have been researched to get good quality CNTs in vertically aligned arrays.71,72 The CVD synthesis employs the use of catalysts in substrates on which nanotubes grow. The metallic nanoparticles are employed as catalysts and their size depends on the diameter of the nanotubes to be synthesized (0.5–5 nm for SWCNTs, 8 to 10 nm for MWCNTs). The nanoparticles Ni, Co, Fe have been used as nano catalysts for the synthesis of CNTs. The CVD reactors use inert gas methane for SWCNT production and ethylene for MWCNTs. In case of SWCNTs, the substrate is heated up to 850 °C–1000 °C and 550 °C–700 °C for MWCNTs synthesis. The thermal decomposition of hydrocarbons produces Carbon which is dissolved in the metal nano catalyst. When a certain concentration of Carbon is attained, its semi-fullerene cap is formed that acts as a basic unit for the growth of the nanotube. The continuous flow of Carbon from the hydrocarbon source to the catalyst particle is maintained. Finally, the CNTs are obtained after purification process and removal of catalysts from the tips and surface of nanotubes. The research is going on for the last step so that high quality of the synthesized material may be obtained.73,74 After the production of CNTs there is a need to purify the material to remove the amorphous carbon materials. Although, arc discharge and laser ablation methods produce SWCNTs in a high quantity but they suffer from drawbacks also as there is a need to evaporate C-atom from solid state source at a very high temperature (>3000 °C) and the nanotubes bundle together during the formation which limits their applications.75–77 The length of CNT depends on the time taken for their growth. The diameter of synthesized SWCNTs varies from 0.7 to 3 nm78 and 10 to 200 nm for MWCNTs.79 The different types of drugs can be effectively loaded on the internal and external surfaces of CNTs due to their large surface area.80,81
Graphene
It is a 2D allotropic form of Carbon comprising of a single layer of Carbon atoms. The Carbon atoms exhibit a hexagonal crystal lattice joined to each other by  and
and  bonds in sp2 hybridization with an interatomic distance of 0.142 nm of Carbon hexagons. The Graphene was first explored, searched by a Canadian theoretical physicist, P. R. Wallace in 1947 whereas the samples were later investigated by a Dutch-British physicist A. Geim and a Russian-British physicist K. Novoselov.82–84 Although, the theoretical investigations on the Graphene have been conducted extensively, the real material has been synthesized only recently. The research on the characteristic features of Graphene is still going on. It do possess extremely high mechanical rigidity and a high thermal stability. The electrical properties of this carbon allotrope basically distinguish from the properties of 3D materials. Graphene is a building block of other allotropes of carbon as it can be wrapped up, rolled up cylindrically or stacked up to get 0D fullerenes, 1D carbon nanotubes and 3D Graphite respectively.85 Thus it depicts the structural element of some other Carbon allotropes such as fullerenes, CNTs and Graphite. The Graphene rolled into 0D buckyballs, 1D nanotube and stacked up into 3D graphite is shown in the Fig. 1.85
bonds in sp2 hybridization with an interatomic distance of 0.142 nm of Carbon hexagons. The Graphene was first explored, searched by a Canadian theoretical physicist, P. R. Wallace in 1947 whereas the samples were later investigated by a Dutch-British physicist A. Geim and a Russian-British physicist K. Novoselov.82–84 Although, the theoretical investigations on the Graphene have been conducted extensively, the real material has been synthesized only recently. The research on the characteristic features of Graphene is still going on. It do possess extremely high mechanical rigidity and a high thermal stability. The electrical properties of this carbon allotrope basically distinguish from the properties of 3D materials. Graphene is a building block of other allotropes of carbon as it can be wrapped up, rolled up cylindrically or stacked up to get 0D fullerenes, 1D carbon nanotubes and 3D Graphite respectively.85 Thus it depicts the structural element of some other Carbon allotropes such as fullerenes, CNTs and Graphite. The Graphene rolled into 0D buckyballs, 1D nanotube and stacked up into 3D graphite is shown in the Fig. 1.85
Figure 1. Graphene rolled into 0D buckyballs, 1D nanotube and stacked up into 3D Graphite, Reprinted with permission from Ref. 85 (Copyright (2007) Springer Nature).
Download figure:
Standard image High-resolution imageGraphene is a semiconductor material with zero band gaps, ambipolar electric field with charge carrier mobility more than 15000 to 20000 cm2 Vs−1 at room temperature. It possesses excellent mechanical, physical, chemical and thermal properties and is transparent to light up to 97.7%. That's why it is a potential candidate for the application in highly sensitive electrochemical sensors.86–89 The mobility of electrons in the layers of Graphene is one hundred times more than that in Silicon.90 That's why it is predicted that one day it will replace Silicon in the electronic industry. Graphene finds immense application in sensors due to its large specific surface area and high charge carrier mobility.91,92 Table II shows the values of significant physical, electronic and mechanical characteristic features of Graphene.
Table II. Significant physical, electronic and mechanical characteristic features of Graphene.
| In plane modulus | ̃1 TPa | 87 |
| Strength | ̃130 GPa | 87 |
| Specific surface area | 2630 m2 g−1 | 93 |
| Thermal conductivity | ̃5000 W mK−1 | 93 |
| Electron mobility at room temperature | ̃105 cm2 Vs−1 | 93 |
Synthesis
The Graphene was firstly isolated in 2004 at the University of Manchester by Novaselov and Geim by isolating individual Graphite layers by applying the peeling off method with a scotch tape.82 This method produces a high quality Graphene devoid of any defect. However, the small size of the sample restricts its use for lab research only and not for commercial applications. The peeling of Graphite in solvents like N-methyl pyrrolidone94 and surfactant sodium dodecyl benzene sulphonate solution95 has been reported. Due to the small fabrication cost and less number of processing steps, this technique has been widely used for the large scale synthesis of Graphene. The layered Graphene has been fabricated by employing CVD peeling off from Graphite,82 the epitaxial route,96 solvothermal production,97 liquid phase exfoliation,94 microwave assisted exfoliation98 and other oxidation techniques.99,100 The Graphene can also be synthesized from the reduction of Graphene oxide but the synthesized material has large number of defects101 whereas the Graphene synthesized from Graphite consist of a small density of defects.102
The CVD technique has been applied for the synthesis of Graphene modified electrodes and devices to be used in electrochemical sensors. The Graphene has also been fabricated by the use of transition metal substrates like Ni,103,104 Po,105 Pt,106 Cu107 on a very large scale. As a consequence of a very low stability of Carbon in Copper, the CVD growth of Graphene over Copper results in a highly crystalline Graphene layers.108 The Epitaxial Graphene can be synthesized by graphitization of doped SiC single crystal wafers at high temperature as well as of undoped crystals of SiC.109,110
Graphene Oxide (GO) has been produced by the chemical oxidation of Graphite at a very low production cost. The Hummers method has been employed for the growth of GO as it takes very small time for the growth and does not dissolve harmful chemicals.99 The synthesis of Graphene oxide (GO) has also been reported by making use of potassium permanganate and concentrated Sulphuric acid as oxidation agent and for peeling off Graphite.99 The acidic treatment accounts for the hydrophilic character of GO. The GO sheets can be dispersed well in water.11 The GO can be reduced back to Graphene by using chemical reduction methods such as by the direct addition of reducing agents like hydrazine111 or by thermal reduction at high temperatures.112
The solution growth of Graphene has been reported to produce GO in which Graphite is oxidized due to which an aqueous colloidal form of GO flakes is produced. As a result, the basal plane of the Graphene is functionalized with hydrophilic functional groups.113–115 GO has a high density of oxygen functional groups (carboxyl, hydroxyl, carbonyl, and epoxy) at its basal plane and its edges due to which it forms a colloidal solution in water and polar solvents and is a novel Graphene material.
Reduced graphene oxide (rGO)
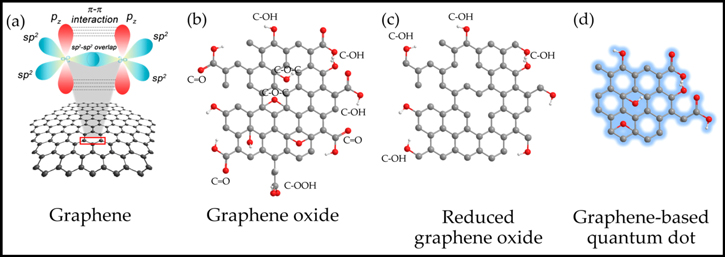
The electrochemical reduction method has also been applied to obtain reduced Graphene oxide (rGO).116 Various reduction methods have been employed to reduce Go partially to form reduced Graphene oxide (rGO) by using laser radiation,117 annealing118 and chemical methods.119 However, the harsh use of chemicals for oxidation degrades the properties of Graphene by damaging the basal plane of the Graphene. That's why peeling off of Graphene from Graphite is done under suitable solvents and surfactants.94,95 The Graphene has a tendency to aggregate into Graphite in some solvents. Thus it is difficult and challenging to prepare pure and uniformly dispersed single layer Graphene in the solvents. The mechanical peeling of the Graphite is done to obtain pure 2D Graphene by making use of the adhesive tapes4 that has lesser density of defects. The structures of Graphene based nanomaterials are shown in the Fig. 2.120
Figure 2. Structures of Graphene based nanomaterials (a) Carbon atoms in pure Graphene with sp2 hybridization (b) Graphene Oxide (GO) (c) Reduced Graphene Oxide (rGO) (d) Graphene Carbon Quantum Dot (GCQD), Reprinted from120 (CC BY-NC 4.0).
Download figure:
Standard image High-resolution imageCarbon nanodiamonds
Another allotrope of carbon, Carbon Nanodiamonds (CNDs) is the nanoparticles with the crystal structure of Diamond, and exhibit excellent properties of diamond.121,122 The CNDs consist of a crystalline Diamond core which is surrounded by a anion like amorphous Graphite shell.123 They do possess very small size, large surface area and large adsorption capacity for the attachment of chemical to biological molecules.124,125 They exhibit exceptional hardness, thermal conductivity, refractive index, coefficient of friction, insulation characteristics and have very low toxicity.126
The CNDs display fluorescence due to the presence of a complex defect N-V, containing nitrogen (N) and a vacancy (V). Since CNDs are chemically stable, their photo luminescent behavior can be used for the several in-vivo and in-vitro applications.127 The CNDs are the potential fluorescent probes for use as biomarkers and in bio labeling studies.128
Synthesis
The CNDs are synthesized artificially by the detonation of explosive to produce these nanoparticles.129 As diamond exhibits fluorescence due to the presence of a complex defect (N-V), containing nitrogen (N) and a vacancy (V), the fluorescent CNDs can be synthesized by doping of N vacancies by means of electron irradiation and annealing in the free space.130 The scientific investigations have reported the fluorescent CNDs consisting of roughly 400 Carbon atoms and Silicon vacancies that have the potential for use in sensing applications.131
The CNDs can be functionalized by the covalent or non-covalent method to provide extra stability to them.132 The covalent modifications of CNDs have produced stable complexes of drugs, whereas with non-covalent methods, the drugs can be easily attached to the CNDs but with decreased stabilities. Since the covalent modifications of CNDs involve complex processes133,134 due to which the non-covalent method is widely used to build CND based drug delivery systems. The colloidal behavior of the nanodiamonds can be enhanced by transforming the surface of CNDs.135 A number of functional bio molecules and drugs have been attached on the surface of CNDs by non-covalent methods.136 The thermal induction and plasma treatment methods have been employed to attach chlorine, ketonic and carboxylic groups on their surface.137–139
The surface modified CND films were used to absorb small molecules like alkyl alcohol, Sulphonic acids, thiols and complex structures like DNA and enzymes.140–144 Using alkyl chains, fluorine and Si, the covalent attachment on the surface of CNDs is feasible.145–147 It has been reported that the ketonic, carboxylic and amino groups can modify the surface of CNDs.148–153 These groups are further changed by a chemical modification in order to functionalize the CND particle. A functionalized CND particle with alkyl group can be distributed in the organic solvents uniformly whereas the non-modified CNDs can be dispersed in water but not in organic solvents. A functionalized CND particle with a Silane coupling reagent can transform a glass substrate.154 The functionalization of CNDs results in the fluorescent behavior without N-V defects. The protein and biotin- supported CNDs were investigated in order to increase their affinity towards the biological molecules. These modified CNDs have the potential to be used for drug delivery systems.155 The fluorescent CNDs can be used for designing nanosensors due to the presence of N-V centers.156 The CNDs can be very easily functionalized with biomolecules after undergoing purification by the ozone gas.157,158
Carbon nanohorns
Carbon Nanohorns (CNHs) are one of the allotrope of Carbon consisting of closet cages of Carbon atoms with a diameter of 2–5 nm and length 40–50 nm.159 They are more beneficial to use than CNTs as they can be synthesized at a larger scale at room temperature without any use of metal catalysts. They can be synthesized by using arc discharge of Carbon rods,160 laser ablation of pure Graphite161 and Joule heating. The CNHs do posses high surface area and good porosity which can be exploited for their potential application in the field of biosensing.161,162
Carbon dots
The Carbon Dots (CDs) are zero-dimensional CNMs consisting of Carbon atoms with a size below 10nm. These materials do possess significant electronic and optical properties as exhibited by Quantum Dots.163 They do possess low toxicity, stability and biocompatibility for their application as electrochemical biosensors.164–166 The CDs have been synthesized by using laser ablation method applied to the Carbon atoms.167 The various processes like pyrolysis,168 hydrothermal synthesis,169 electrochemical methods170 and microwave synthesis171 have been used to synthesize CDs. They can also be prepared by using the soot of the candle flame.172
CDs can be classified into Carbon Quantum Dots (CQDs) and Graphene Quantum Dots (GQDs). The CQDs and GQDs have a diameter range from 1 to 10 nm. The GQDs consist of Graphene layers of size less than 10 nm. They can be synthesized by using thermal plasma jet technique with low fabrication cost. They can be an alternative to the nanodiamonds.173–176
Carbon nanofibres
Carbon Nanofibres (CNFs) are cylindrical wire shaped nanostructures in which graphene sheets are piled in different arrangements such as ribbon-like, platelet or herringbone. The length of CNFs varies in order of micrometers and can be up to 10 μm whereas their diameters vary from 10 to 500 nm. Their mechanical strength and electric properties are just like that of CNTS.177 As a consequence of stacking of graphene sheets with different shapes in different arrangements, CNFs have more edge sites on their outer walls in comparison to CNTs. The presence of edge sites makes it feasible to transfer electrons with electro active species in solution and the detector substrate.178,179 The CNFs do possess attributes like good electrical conductivity, large surface area, biocompatibility and easy fabrication process that are vital for electrochemical sensing applications. Moreover, CNFs can be easily functionalized to suit a particular detection mechanism.
Synthesis
The CNFs can be prepared by employing arc discharge180 and laser ablation181 methods. The thermal chemical vapour deposition (CVD),Plasma enhanced chemical vapour deposition(PECVD) and electro spinning have also been employed for the preparation of CNFs.182 During thermal CVD method, a compound containing hydrogen and carbon is thermally decomposed by employing a metal catalyst at a constant temperature.183 This method is further divided into three types based on the way in which the catalyst is employed i.e. Substrate method, Spray method and Gas phase flow catalytic method.
During PECVD method, the high energy electrons present in the plasma collide with the gas molecules. As a result, they transfer their kinetic energy to them, thereby, causing excitation, ionization and decomposition which results in production of CNFs.184–186
During electro spinning process, the polymers like silk, DNA, collagen and polyester have been used to obtain CNFs. The polymeric solution is firstly subjected to a potential of very high volts for getting charged, then to a spinning port where it is moved at a very fast rate. As a result, the nanofibres get deposited at the collecting plate in the form of a mat. The fiber mat undergoes oxidation and is carbonized in nitrogen atmosphere to produce CNFs.187–190 The CVD produces CNFs with impurities which require a further complicated purification process whereas electro spinning produces CNFS through a very easy process with high purity.191,192 The Fig. 3 shows the SEM images of alignment of CNFs grown on a Silicon substrate in plasma growth process in the presence of electric field (a) & (b) and CNFs exposed density (c).193
Figure 3. SEM images of CNF array. (a) & (b) Alignment of CNFs grown on a Silicon substrate in plasma growth process (c) CNFs exposed density, reprinted with permission from.193
Download figure:
Standard image High-resolution imageCarbon black
The Carbon black (CB) is a nanomaterial prepared from the combustion of petroleum products. They are the nanoparticles spherical in shape and are strongly bonded to each other to form aggregates. The size of Carbon black particles varies from 3.0 to 100nM. The significant physical, electronic and mechanical characteristic features of Carbon black are given in Table III. The conductivity of Carbon black can be enhanced by heating up to 7000 °C because more number of electrons in sp2 hybridization state with delocalized pi-bonds is available for the conduction of current.194 Due to their large surface area; a large number of oxygenated groups are formed at the edges of the Carbon black nanoparticles. It is the presence of sp2 hybridized Carbon atom edge planes and oxygenated groups over the Carbon black nanomaterials that make them capable to attach biomolecules on their surface to act as electrochemical biosensors. They can be used for the detection of analytes for the biosensing applications.
Synthesis
The Carbon black nanoparticles can be prepared by employing furnance, channel and acetylene processes.201,202 The preparation process is very easy and has low cost. The properties of Carbon black can be easily tailored by introducing other materials such as polymers203 or metallic nanoparticles into them for better electrochemical sensing applications.204
Biosensors
Biosensors have been extensively used for the detection of biological molecules, pathogens and other disease causing agents in the healthcare field. The biosensors are basically chemical sensors which make use of the recognition properties of the biomolecules in its sensitive layer. The CNMs have been extensively used for providing immobilization aid to the recognition molecules in the biosensors. A typical biosensor consists of three parts (i) a recognition molecule that can be an enzyme, protein, antibody or DNA etc. (ii) a transducer element which records the interaction as a signal between the analyte or target and the recognition molecule (iii) a signal processor. An electrochemical biosensor is attached with sensitive biological molecules on the surface of solid electrodes by employing recognition properties of biomolecules in order to hold the target molecules on the surface of electrode. As a result of this process, a reaction signal is converted into an electrical signal like voltage, current, impedance etc. which can be easily detected. Most of the biosensors that have been developed are electrochemical in nature. The electrochemical biosensors are potentiometric, amperometric or conductometric depending upon the signal generated from the electrochemical process which can be a resistance, current or voltage signal respectively. The electrochemical biosensors have been used to study the qualitative as well as the quantitative aspects of the detected molecule. The electrochemical biosensors are highly sensitive to ensure detection, highly selective to avoid the interference of other species, small in size, easy to use and cost effective.205,206
Why CNMs for Electrochemical Biosensors
The CNMs have been extensively used for the electrochemical biosensors due to their large surface area due to which many detection events can occur simultaneously on their surface and also, the attachment of the biomolecules is possible very easily. These materials have such electronic, optical, physical and mechanical properties which make them potential candidates for use in biosensors. Their charge storage and electron transfer properties can be engineered for the electrochemical applications. These materials have low cost, wide potential range over which the CNMs electrode can operate, high electro catalytic activities for a large number of redox-active chemical and biological systems. The electrochemical performance of the biosensors can be optimized by modifying the structure of these molecules to engineer their electronic, chemical and crystalline properties for a particular application. The CNMs based surfaces can be easily tailored by means of functionalization by a large number of covalent and non covalent methods which enhances their electrochemical sensing capabilities. These materials are also highly biocompatible. The CNT sensors do posses the ability to transport electrons faster, highly sensitive and are capable of detection even at very low limits. The Graphene has also been effectively used for the electrochemical sensing due to their significant electron transport features as described in the Table no I and II. The CNT or Graphene based electrochemical sensors have higher sensitivity, higher selectivity, fast electron transfer rate and low limits of detection. The doping can significantly influence the electronic, mechanical and conducting properties of CNTs. Not only this, the distinct forms of CNMs have a varying density of states. The density of states of CNMs based electrode determines the electron transfer capabilities with the target molecules. For a faster electron transfer process, the energy of electrons in the electrode should be equal to that in the redox reaction. A higher density of state enhances the possibility of existence of electrons with enough high energy needed for their transfer to the redox system.207 The density of states for CNMs varies with their structure and can be adjusted by making changes in their atomic bonding structures. It also depends upon the tube diameter in case of CNTS. The density of states can be increased by peeling off CNTs in a controlled manner.208
The controlled oxidation of MWCNTS can enhance their electrochemical performance by modifying their electronic structure. The inorganic particles can be strongly chemically coupled to the CNMs to change the electronic structure of each individual component to provide synergistic electro catalytic activities to the resulting hybrid systems.209
The planar geometry of Graphene and tubular geometry of nanotubes makes it possible to expose the surface atoms for forming chemical bonds with a large number of molecules of the target material for the biosensing applications. Every atom on the surface of Graphene is exposed due to its high exceptional surface area. Thus, the high molecular functionalization is feasible in Graphene in comparison to other carbon nanomaterials.
The CNMs do possess a high surface to volume ratio, electrical conductivity and mechanical strength that makes them potential for use in electrochemical biosensors.210–212 The large surface area electrical, thermal conductivity and strength of CNTs make them suitable for use in electrochemical biosensors.51 The basal planes of Graphene play a major role in electrochemical process than the edge planes.213,214 The Carbon nanostructures have the outstanding photo thermal response. The photo thermal technique has been used to reduce/eliminate the size of tumors.215,216
Carbon Nanotubes as Electrochemical Biosensors
The CNTs have been widely explored for their use in the electrochemical sensing of biomolecules for various biomedical applications as shown in the Fig. 4.217–220 The properties of CNTs can be customized to suit their potential as biosensors. The antibodies and enzymes can customize the features of CNTs in the electrochemical biosensors. The characteristics of CNTs as biosensors can also be tailored by the peptides and nucleic acids as they do have the inherent capability to be acquainted with bio-elements or biomolecules. The method of analysis i.e. invivo or invitro determines the design of a biosensor. The biomolecules are attached on the surface of CNTs in order to prepare the surface for a particular detection process. The various biomolecules such as enzymes, proteins or nucleic acids have been extensively used in the CNT biosensors for this purpose.
Figure 4. CNTS as biosensors for different applications, Reprinted with permission from221 (Copyright 2014, Elsevier).
Download figure:
Standard image High-resolution imageCovalent or non-covalent functionalization of CNTS
The physical and chemical properties of nanoparticles can be engineered by subjecting them to functionalization, by attaching some molecules on their surface.222 The CNTs are not soluble in aqueous solutions but when they undergo oxidation in a mixture of acids, the carboxylic groups attach to the surface and side walls of the nanotubes making them soluble in aqueous solutions. Thus, functionalization has proved to be a boon to the CNTs for modifying their physical and chemical properties.222,223 The functionalization of CNTs with chemicals or suspension in a surfactant containing solution results in decrease in their bundle formation.224
The most commonly used tailoring technique for CNTs in order to enhance their electrochemical sensing performance (sensitivity and selectivity) are covalent or non-covalent functionalization. The biomolecules are attached to the surface of CNTs by covalent or non-covalent functionalization. In covalent functionalization, the various chemical functional groups like carboxylic and amine groups are attached to the surface and side walls of CNTs by certain chemical processes. These functional groups on the CNTs react with the functional groups present in the bimolecular structure resulting in the formation of a covalent or a non-covalent bond. The CNT functionalized with poly (amidoamine) dendrimer through covalent functionalization has been used for the attachment of glucose oxidase and Horseradish peroxidase.225 The use of covalent functionalization for the attachment of biomolecules on CNTs for sensing glucose,225,226 H2O2,227 aflatoxin,228 carcinoembroyonic antigen detection229 have been reported. The amine functionalized CNTs interact with the amino groups on biomolecules such as enzymes, proteins and nucleic acids. The glutaraldehyde, active ester or epoxy has been used to attach the amine containing biomolecules to the CNTs.226,229
An electrochemical immunosensor has been developed for the detection of carcinoembryonic antigen in saliva and serum in which monoclonal anti-carcinoembryonic antigen antibodies are attached on polyethylene amine treated MWCNTs side walls by using covalent functionalization with the help of glutaraldehyde.229
The vertically aligned SWCNTs are attached on a GCE with a covalent bonding230 or on a gold surface by diazonium has been studied and investigated. The SWCNTs modified with diazonium have displayed the highest electron transfer in cellobiose hydrogenase from phanerochaete Sordida with small values of lactose oxidation potential.231
However, the covalent functionalization of CNTs has an influence on its intrinsic properties as the change in CNT surface by covalent attachment can cause hybridization to change from sp2 to sp3. As a consequence of it, the mechanical strength and electrical properties could be hampered due to the decrease in conjugation abilities of the CNTs.232,233
The non-covalent functionalization of CNTs has been significant for attaching the biomolecules on CNTs as it does not affect the intrinsic properties of CNTs. As a consequence of that the mechanical and electrical properties are not affected.232,233 The CNTs are non-covalent functionalized as a result of pi-pi electrostatic interactions between the CNTs and the biomolecules.233,234 The adsorption of aromatic molecules and benzene derivatives on the surface of SWCNTs has been achieved due to the establishment of pi-pi interactions between the CNTs and the benzene derivatives.235 The aromatic compounds have been employed for attaching the biomolecules on the surface of CNTs by non-covalent functionalization such as ferrocane,236–238 anthracene,239–241 pyrene242–244 etc for the development of electrochemical biosensors. The non-covalent functionalized CNTs by aromatic compounds have been used for the bioelectrocatalysis of oxygen,239,242,245–248 glucose biosensors,236,237,249 H2O2 detection,250 ethanol biosensor,251 and trichloroacetic acid biosensor.252 The polymers have been employed for the non-covalent functionalization of CNTs for use in biosensors. The polymers like polypyrroles,253,254 glycolipids,255,256 polyethyleneimine257–260 have been used for the non-covalent functionalization of CNTs. The commonly used tailoring techniques for CNTs have been functionalization with conducting polymers,261–263 mixing with surfactants or polyelectrolyte,264–266 using metal oxides or nanoparticles,267–270 adding enzymes56,266,271–275 and doping with heteroatom.55,276
Detection of dopamine
The first use of CNT electrochemical biosensor was reported in 1996 where it was used for the detection of dopamine.220 The CNT biosensor exhibited better detection in comparison to other Carbon based sensors due to the fact that there was the presence of pores due to the packaging of CNTs as a result of which its surface area was increased for the attachment of dopamine.277 The formation of oxygen containing groups at the surface of nanotube during oxidation process has also a significant effect on the detection process with CNTs. The multi-walled carbon nanotubes have been mixed with bromoform to obtain a paste which is packed into a glass tube forming a CNT Carbon paste sensor. The CV and DPV characterization techniques have been employed to study the oxidation of dopamine by the traditional Carbon sensor and CNT based carbon paste sensor.220
The dopamine has also been detected in the presence of ascorbic acid by employing MWCNTs functionalized with Sodium dedecyl sulphate on a Ta substrate. The DPV was employed to detect the dopamine in the presence of ascorbic acid. However, the detection levels were in the micrometer range i.e. limit of detection is 3.75 μM for dopamine in the presence of ascorbic acid with range from 0.02mM to 0.2 mM which restricts the applicability of the sensor for the practical use.264 The modification of a MWCNTs electrode with RuO2 has an influence on the enhancement of limit of detection using DPV than the MWCNTs. A much higher concentration of dopamine, ascorbic acid and uric acid in the μM to mM range have been tested using this investigation and the electrode is highly selective to the presence of dopamine, ascorbic acid and uric acid.267
The Over Oxidized polypyrrole (OPPY)-MWCNT-modified Glassy Carbon Electrode (GCE) has reported an enhanced sensitivity to the detection of dopamine. The electrochemical deposition has been employed to tailor MWCNTs with cadmium oxide (CdO) nanoparticles of size approx. 50 nm diameters. The resulting CdO/MWCNT sensor does not exhibit any signal response to uric acid, ascorbic acid and dopamine up to 100 μM but it has a better selectivity for H2O2.269 The enzyme laccase has also been used to detect the presence of dopamine in the presence of ascorbic acid and 3, 4-dihydroxyphenyl acetic acid (DOPAC). In this technique, the limit of detection of 0.4 μM has been reported with high selectivity.271 The Tyrosinase and Nafion has been employed for the detection of dopamine by using MWCNTs but the limit of detection was reported to be very low of 0.5 μM.266 The doping of CNTs with certain elements can customize their electronic properties, conductivity and mechanical strength.276 The doping of CNTs with Boron for the detection of dopamine is significant due to the presence of more functionalized groups at the defect sites of CNTs and presence of extra edge plane sites.55 The limit of detection of 1.4 nM has been reported in Boron doped CNTs which has been enhanced by incorporating Boron in CNTs. The SWCNTs functionalized by over-oxidized polypyrrole has been electro-copolymerized on the surface of the electrode.262,263 The films of over-oxidized polypyrrole allowed the cations of dopamine to pass through them but repelled ascorbic acid and serotonin thus exhibiting better selectivity.261 The use of untreated DWCNT has been reported to be the best choice for the development of amperometric sensors. The CV, XPS and BET analysis have shown that the smaller length DWCNTs are more effected by the acid functionalization which decreases the electro active area of the respective modified electrode due to which the amperometric sensitivity in the detection of dopamine and catechol on the functionalized DWCNTs modified electrode is decreased.278
The MWCNTs, SWCNTs, GO, rGO and CQDs have been employed for the electrode modification in the detection of dopamine. However, due to the presence of one type of nanostructures, these materials almost provide same sensitivity and linear range of detection for dopamine. The linear range and lower limit of detection can be improved by employing the hybrid or composite materials of Carbon nanostructures.279 The electrochemical functionalization plays a very important role in increasing limit of detection and sensitivity in dopamine sensor with the SWCNT electrode. The limit of detection of approx. 100 nM and 1 μM has been reported for the functionalized electrode and as-fabricated electrodes respectively. The functionalized SWCNT electrode exhibited better selectivity to dopamine in the presence of ascorbic acid and uric acid than the as-fabricated electrodes.280
CNT based glucose biosensors
The CVD synthesized CNT fibers has been used as a sensing electrode to detect the presence of glucose. The CNT based biosensors have exhibited a very fast amperometric response to the presence of glucose.281–283 The glucose based CNTs have been extensively investigated and reported.284
A glucose biosensor has been fabricated by using phase separation method by employing MWCNT-grafted chitosan (CS)-nanowire (NW) to which glucose oxidase is attached to obtain the biosensor. The electrochemical detection of glucose was done by employing cyclic voltammetry and amperometry. The fabricated biosensor exhibited a high sensitivity of 5.03 μA/mM in a concentration range of 1–100 mM and a low response time to the detection of glucose. The MWCNTs-CS-NW facilitates the conduction of electrons between glucose oxidase and target molecules.285 In another study, Glucose oxidase was attached on CNT nanoelectrode ensembles by covalent bonding with the formation of amide linkages between their amine residues and the carboxylic acid groups present on the tips of CNTs. The release of H2O2 from the enzymatic reaction of glucose oxidase upon the glucose and oxygen on CNT nanoelectrodes causes the detection of glucose.286
In another study, a biosensor employing Ni-nanoparticles dispersed in vertically aligned CNTs grown on Si/SiO2 substrate has been reported in which the Ni-nanoparticles have deposited uniformly inside and on the top of the CNT forest. The fabricated biosensor exhibited a sensitivity of 1433μAmM1cm2 with a limit of detection 2 μM over a linear range between 5 μM to 7 μM.287 In another study, CuO nanoparticles deposited on the side walls and tips of vertically well-aligned MWCNTs array by a two step electro deposition method has been investigated. The CuO-modified MWCNT electrochemical biosensor exhibited a limit of detection of 800nM and a very high sensitivity of 2190 μAmM−1cm−2 with a linear range response up to 3.0mM glucose concentration with rapid response and good stability.288 The Pt nanoparticles loaded CNTs composites have been prepared under hydrothermal conditions by polymerization reaction of glucose and reduction deposition of a platinum source in the pores of anodic alumina membranes. The fabricated electrode has been used as a amperometric sensor for the low potential detection of H2O2. The as-prepared Pt-CNT glucose biosensor displayed a limit of detection of 0.055mM with a linear range of 0.16–11.5 nM glucose concentration with a very high sensitivity and selectivity.289 Another CuO-MWCNTs fabricated biosensor exhibited a high sensitivity of 2596 μAmM−1cm−2 to the detection of glucose with a limit of detection of 0.22 μM in a linear range over a concentration up to 1.2 mM.290
CNT based enzymatic biosensors
The CNT based electrochemical biosensors have been fabricated by using dehydrogenase enzymes such as alcohol-dehydrogenase,291 phosphatase,292 D-fructose dehydrogenase and glucose dehydrogenase. A biosensor employing alcohol-dehydrogenase enzyme along with MWCNT and poly (vinyl alcohol) has reported a response time of about 8 s for the ethanol detection. The electro oxidation of NADH which is produced during the enzymatic activity produces a current that is taken into account for generating the response by the biosensor.291 Other electrochemical biosensors have been fabricated by using acetyl cholinesterase, alkalinephosphatase, organophosphorus hydrolase and urease enzymes. The voltammetric biosensors have been fabricated by attaching urease and acetyl cholinesterase enzymes on the surface of the SWCNT modified electrode by means of sol-gel material for the detection of urea and acetylthiocholine respectively.291 In another study, alkaline phosphatase enzyme is attached on the surface of CNTs in the presence of streptavidin by using layer by layer method.293 The lactate level monitoring is significant for the use in biotechnology, food processing and in sports medicine. The amperometric detection of the level of lactate has been done by using CNT and mineral oil paste having lactate oxidase.292
The electronic structures of SWCNTs have an influence on the detection in CNTs enzymatic biosensor. The [FeFe]-hydrogenase enzyme from clostridium acetobutylicum when attached to metallic SWCNTs have reported better electro catalytic activity.273 This is because of the better coordination between clostridium acetobutylicum redox-active sites and the electron surface.273 The glutamate hydrogenase was attached to the top of CNTs by covalent bonding to fabricate a glutamate biosensor that has been able to display the limit of detection up to 10 nM. The CNT electrodes are better candidates for the fabrication of enzymatic biosensors with a high sensitivity and high selectivity.274
An electrochemical biosensor for the determination of tyrosine has been reported. The sensor was fabricated by dissolving polysulfone (PSF) in dichloromethane and depositing it on a GCE. The nitric acid functionalized MWCNTs are drop coated on the PSF layer. The Tyrosinase enzyme (TyOx) is deposited on MWCNT/PSF/GCE after cross linking with glutaraldehyde. The characterization of the biosensor has been done by using the CV and electric impedance spectroscopy. The biosensor exhibited a very low limit of detection 0.3 nM and a very high sensitivity 1.988 μAμM−1cm−2 to detect the tyrosine.294 The doping of CNTs by nitrogen has an influence on the electrochemical performance for the detection of H2O2 and has been used to make H2O2 based enzymatic biosensor at a lower potential.295,296
The Hydrogen peroxide biosensors have been fabricated by using enzyme horseradish peroxidase(HRP) in which the enzyme is attached on the surface of MWCNTs with the aid of mediator methylene blue and by crossing linkage between HRP and BSA composite film.297,298 A hydrogen peroxide detector has been reported by employing CNTs modified with Pt nanoparticles fabricated by means of chemical reduction method on the surface of a waxed graphite electrode.299 The glucose oxidase was attached on the side walls of SWCNTs in order to detect the presence of glucose. The glucose oxidase attached SWCNTs exhibited an enhancement in conductance upon adding glucose and thus acting as a biosensor for the enzymatic activities.56
Detection of proteins
The cellular prion protein has been detected by making use of a fluorescent-label aptamer which involves the quenching of the fluorescence by non-covalent modification of the MWCNTs with the aptamer. But the quenching is restored in the presence of cellular prion protein. The biosensor exhibited a limit of at least 4.1 nM. The sensor demonstrated outstanding selectivity for the cellular prion protein in the presence of amino acid and other proteins.300 The SWCNTs optical biosensor has been reported for the detection of protein-protein interaction with limit of detection 10 pM.301 The insulin has been detected by SWCNTs upon functionalization with insulin-binding aptamer.302 The CNT electrochemical biosensors for the detection of nucleic acids have been investigated vastly and have been brought in notice.303–308
Detection of prostate specific antigen
A highly sensitive electrochemical biosensor based on CNT-bioconjugates and SWCNT forest platform has been reported for the detection of prostate specific antigen (PSA) detection by using horseradish peroxidase (HRP)/secondary antibody ratio. The device exhibited a limit of detection of 4 pgmL−1 in 10 μl calf serum without any dilution.309 With the replacement of SWCNTs with gold nanoparticles customized MWCNTs, the limit of detection of PSA changed to 0.40 pgmL−1,thereby, significantly enhancing the sensitivity and selectivity of detection.310 The CNT based biosensors have been coated with the antibodies for the detection of the biomolecules/disease causing agents. The CNTS-arrayed electrodes coated with anti-PSA antibodies have been reported to detect PSA.311 The immunosensors have been reported for the detection of osteopontin that causes prostate cancer. They have been fabricated on the glass substrate which is coated with SWCNTs with attached osteopontin molecules on their surface.312 Another biosensor, A CNT (EDC/NHS functionalized) transistor and an antibody which is a hybrid to the genetically engineered antibody have been used for the detection of osteopontin.313 The DNA strands functionalized MWCNTs and SWCNTs have been reported for the detection of PSA in the blood samples with more sensitivity and selectivity.314 The electrochemical biosensors used for the detection of prostate cancer biomarker using nanoparticles have been briefly reviewed and reported.315
Detection of blood cholesterol level
The amount of blood cholesterol level has been detected by the amperometric electrochemical biosensors.316–318 The amperometric biosensors employing sol gel chitosin/silica and MWCNTs organic-inorganic nanohybrid composite material has been reported for the detection of cholesterol in the blood.318 The cholesterol oxidase enzymes are used for the oxidation of cholesterol to 4-cholesten-3-one producing hydrogen peroxide. The electrochemical detection of level of H2O2 produced during the enzymatic activity gives the level of cholesterol in the blood. An amperometric cholesterol biosensor has been fabricated through layer by layer deposition of (poly (diallyl dimethyl ammonium) chloride) and cholesterol oxidase enzyme on a MWCNT electrode modified by the gold nanoparticles. A protective coating of non conducting (poly (o-phenylenediamine) film has been produced over it by means of the electrochemical methods. A limit of detection of 0.2 mM to the detection of cholesterol has been reported by the fabricated biosensor.316
Detection of cancer cells
A poly dopamine coated CNT functionalized by the folic acid has been used for the detection of HeLa and HL60 cancer cells.319 The biosensors have been reported to detect O2 released from HeLa cells which have been fabricated from the hollow Carbon cubic (HCC) and porous Carbon cubic (PCC) nanomaterials. The etching of zeolitic imidazolate framework K-8(ZIF-8) with tannic acid followed by calcinations process produces HCC nanomaterials whereas PCC nanomaterials were obtained by direct pyrolysis of ZIF-8. The HCC and PCC are attached on the surface of Screen printed Carbon electrode (SPCE). The HCC based sensor have exhibited a limit of detection 207 nM whereas the PCC electrode have reported a limit of detection 140 nM to detect the O2 released from HeLa cells. The selectivity of the biosensors has been examined by studying the amperometric response in the presence of interfering agents like 4-acetaimidophenol, uric acid, ascorbic acid, D-glucose and dopamine. The biosensors displayed very small current response to them, thereby, exhibiting excellent selectivity.320 The synthesis of Hollow Carbon Cubic (HCC) and Porous Carbon Cubic (PCC) nanomaterial and various steps involved in the detection of superoxide anions in HeLa cell are shown in Fig. 5.320
Figure 5. Synthesis of Hollow Carbon Cubic (HCC) and Porous Carbon Cubic (PCC) nanomaterial and various steps involved in the detection of superoxide anions in HeLa cell, Reprinted with permission from320 (Copyright (2019) Springer Nature).
Download figure:
Standard image High-resolution imageThe surface ITO electrode coated with an assembly of CNT multilayer and antibodies to the epithelial cell adhesion molecules have been used to detect the cancer cells of liver.321 The Au-Ag alloy coated MWCNTs have been used for the detection of volatile biomarkers of the gastric cancer cells MGC-803.322 The femtomolar level gastric cancer biomarker miRNA-106a has also been detected and reported.323
The carcinoembryonic antigen has been detected by the CNT based electrochemical immunosensor employing gold nanoparticles- encapsulated dendrimers as a sensor surface with the attachment of two enzymes Glucose oxidase and horseradish peroxidase. The electron transfer is facilitated by the encapsulation of gold nanoparticles in the interior structure of the device. The immunosensor has exhibited a wide concentration range from 10 pgmL−1 to 50 ngmL−1 and the limit of detection has been reported to be lower as compared to the ELISA test.324
Detection of DNA hybridization
A DNA hybridization biosensor has been reported based on the ssDNA and SWCNT. The ssDNA was wrapped over the SWCNT by the hydrophobic interaction. The DPV response detection technique has been applied during the DNA hybridization process. The detector exhibited a low response between 40–110 nM range and limit of detection 20 nM.310 The hybridization of DNA targets have been examined by the CNT modified electrode for the DNA analysis with Ru(bpy) 2+ mediated guanine oxidation. By using this method, the hybridization of less than a few attomoles of oligonucleotide targets has been detected. The Fig. 6 shows the fabrication of DNA/Self assembled MWCNTs electrodes for the detection of target DNA sequences.325 An electrochemical DNA sensor using hollow carbon spheres (HCS) decorated with polyaniline (PANI) has been reported in which thiolated 21-meroligonucleotide is attached by the electrodeposited gold nanoparticles on the HCS-PANI substrate. The biosensor has reported a limit of detection 10fM for the detection of the target sequences of Hepatitis B virus DNA. The selectivity of the biosensor has been studied by using different types of DNA strands which comprises of a non complementary strand and a mutated one. The DNA sensor has exhibited more selectivity to the complementary strand than the other ones.326
Figure 6. The fabrication of DNA/Self assembled MWCNTs electrodes for the detection of target DNA sequences, Reprinted with permission from325 (Copyright 2004, Elsevier).
Download figure:
Standard image High-resolution imageAn electrochemical sensor employing SPCE with 1w% MWCNT and 1w% Graphene has been reported for the detection of H2O2 and nicotinamide adenine dinucleotide (NAD+/NADH) and has exhibited a limit of detection of 7.1 μM and 3.6 μF for the detection of H2O2 and NADH respectively with sensitivity 0.0027 μAμM−1 and 0.0075 μAμM−1 respectively.327
The DNA aptamer based biosensors has been employed for the detection of metal ion, organic dyes, peptides, proteins, cancer cells and infectious microorganisms.328 The DNA aptamers are the potential candidates for the biosensors as they do have small size, low cost and large stability.329 The Au-electrodes coated by MWCNTs have been used as biosensors for detecting the target DNA sequences. The DNA probes are adsorbed on the self assembled MWCNTs and methylene blue has been used as an indicator. The change in the voltammetric peak of the methylene blue has been used to detect the hybridization of DNA on the electrodes with more selectivity.330 The CV has been used for the detection of DNA with ferrocene labeled complimentary Oligonucleotide which gives reversible electrochemical response.331 The hybridization of the DNA on a DNA- functionalized CNT array has been detected by the DPV with daunomycine as a redox label agent.332 An electrochemical micro fluidic DNA sensor has been fabricated by employing carboxyl functionalized MWCNTs. The working and counter electrodes have been developed through ITO by using wet chemical etching technique. The working electrode was modified by using electrophoretic deposition of MWCNT and attachment of a DNA probe. The detector exhibited detection at a concentration as low as 1 fM with a response time of 1 min to detect the oligonucleotide. The modification by MWCNTs resulted in enhanced surface area of the electrode for loading DNA which leads to higher sensitivity and higher linear range. The biosensor has exhibited the ability to distinguish mismatch in single nucleotide and non complementary DNA sequence, thereby, exhibiting very good selectivity.333 An electrochemical DNA biosensor using ITO modified with curcumin have been reported to treat the Alzheimer's and artery coronary diseases by detecting APOe4 DNA.334 The various steps involved in the fabrication of electrochemical DNA biosensor employing GQDs as the surface modifier of the ITO electrode to sense Alzheimer's and artery coronary diseases are shown in the Fig. 7.334
Figure 7. Various steps involved in the fabrication of electrochemical DNA biosensor employing GQDs as the surface modifier of the ITO electrode to sense Alzheimer's and artery coronary diseases, Reprinted with permission from334 (Copyright 2018, Elsevier).
Download figure:
Standard image High-resolution imageThe CNTs can also be used as an optical biosensor for the detection of damage to the DNA. The fluorescence response of DNA tailored SWCNTs have been researched and reported. The DNA coding at the surface leads to a strong luminescence. However, the luminescence gets decreased upon the damage to the DNA. Thus, the light emitted from the nanotube can be correlated with the concentration of the DNA damaging agent by making use of principal component analytical method.335
Detection of nitride (NO2−) and trichloroacetic acid
The GCE modified with nafian coated MWCNTs and mesoporous silica MCM41 nanoparticles functionalized with hemoglobin(Hb) hybrid conjugate has been employed as a biosensor for the detection of nitride (NO2-) and trichloroacetic acid (TCA) exhibiting higher hypersensitivity and selectively which is attributed to the exceptional biocatlytic activity of Hb and excellent charge transfer capability of the heme group. The biosensor did not exhibit any amperometric response to the addition of cations, anions and biomolecules, thereby, exhibiting very good selectivity.336 The various steps involved in the preparation of MWCNTs-MCM41-Hb hybrid bioconjugate are shown in the Fig. 8.336
Figure 8. Various steps involved in the preparation of MWCNTs-MCM41-Hb hybrid bioconjugate, Reprinted with permission from336 (Copyright 2018, Elsevier).
Download figure:
Standard image High-resolution imageDetection of E. Coli
An electrochemical phage based biosensor has been reported for the detection of E. coli by employing Polyethyleneimine (PEI)-functionalized CNT. The positively charged PEI-CNT facilitates to place the phage particle in the right orientation on the electrode. The phage detector exhibited a limit of detection of 103 CFUmL−1 and a range in between 103 CFUmL−1 and 107 CFUmL−1.337 The attachment and orientation of bacteriophages onto PEI-functionalized CNT on the surface of electrode and the SEM images of CNT without and with PEI-functionalization is shown in Fig. 9.337
Figure 9. The attachment and orientation of bacteriophages onto PEI-functionalized CNT on the surface of electrode and the SEM images of CNT without and with PEI-functionalization, Reprinted with permission from337 (Copyright 2017, American Chemical Society).
Download figure:
Standard image High-resolution imageGraphene Based Electrochemical Biosensors
The application of Graphene based nanomaterials for the electrochemical biosensing have been widely reported. The GO can be a potential candidate for use in sensors.338 Therefore; Graphene and GO nano-sheets have been engineered for the detection of biomolecules and as efficient nano carriers for the drug delivery systems.339,340 The multilayer Graphene nanoflake films have been grown on the Silicon substrates without any use of catalysts. These films contained Graphene sheets stacked with each other to a thickness of several tens of nanometers. The Graphene electrode has exhibited a better performance for the detection of dopamine in the presence of ascorbic acid and uric acid in comparison to that of GCE.341 The Graphene exhibits a better potential for the electrochemical detection of dopamine and serotonin as compared to SWCNTs as it has reported a better performance than SWCNTs in the detection of dopamine in the presence of ascorbic acid and serotonin.342 The performance of Graphene based electrochemical sensors has been enhanced by using oxygen plasma treatment that results in the generation of oxygenated functionalities, edge plane sites and defects. The treated films have exhibited a better detection response to the dopamine, ascorbic acid, uric acid and NADH.343
It has been reported that the metallic impurities present in the CNTs can have toxic effects344 whereas the Graphene is non-toxic in nature, so it is more suitable for use in bio-sensing applications.345 The Graphene is synthesized from graphite which is not very costly whereas the CNTS are synthesized from Carbon containing gas in which nanoparticles are used as templates. The Graphene do posses a large surface area for the attachment of biomolecules and more uniform distribution of active electron sites than those in CNTs. The electrochemical sensing features like sensitivity and selectivity can be enhanced by customizing the properties of Graphene by various methods such as Oxygen plasma treatment,343 formation of Graphene-inorganic oxide hybrids,346–348 doping of Graphene with heteroatom,331,349–351 covalent bonding with dienophiles352,353 and non-covalent bonding with pyrene derivatives.354,355
Detection of glucose
The first Graphene based electrochemical biosensor for the detection of glucose was reported in 2009. The device displayed a limit of detection in the range of 2–14 nM and has highly stable output. Another biosensor employing the attachment of Glucose Oxidase in a Graphene-chitosan nanocomposite has been reported which has detected glucose in a range from 0.08 mM to 12 mM with greater sensitivity and selectivity.356,357
The Horseradish peroxidase (HP) and lactate oxidase have also been attached on the surface of Graphene which have displayed better electrochemical performance.358,359 A porous Graphene biosensor has been fabricated by the attachment of catalase enzyme.360 The in-situ detection of the glucose and urea within the same sample has been made possible by modifying GCE surface by the chitosan-rGO complex. The pH of the sample gets increased by the oxidation of urea by urease and suppressing the electron transfer of Fe(CN)63- whereas pH is decreased by the oxidation of glucose by Glucose Oxidase, making it feasible to transfer electrons in Fe(CN)63−.361 A glucose oxidase enzyme based biosensor using spatially separated electrochemically reduced Graphene oxide (ERGO) by MWCNTs functionalized with 4-(pyrrole-1-yl) benzoic acid has been reported for the detection of glucose in real food samples. Through CV experiments, it was reported that the presence of ERGO in the conductive material increase the rate of electron transfer between the enzyme redox centre and the electrode surface.362
Detection of DNA
The detection of DNA plays a pivotal role in the diagnosis of various genetic or mutated medical ailments in the body. The electrochemical biosensor employed for detection of DNA has attracted the attention of researchers due to its low cost, high sensitivity and high selectivity for the detection of DNA sequences or mutated genes responsible for the various human diseases. The electrochemical DNA sensor working on the principle of direct oxidation of DNA is the simplest one. The Graphene has been used for the direct oxidation of DNA. The Graphene electrode has displayed the well resolved oxidation current signals of the free bases which are guanine, adenine, thymine and cytosine on DPV. Thus the Graphene electrode has displayed a higher electro catalytic activity to the oxidation of DNA. The electrochemical signals for the oxidized single stranded and double stranded DNA at the Graphene electrode have been well resolved.363 The rGO nanowalls deposited on a Graphite rod by electrophoretic deposition method has also been employed for the detection of four bases of DNA which displayed the highly resolved oxidation signals for the four bases of DNA.364
The anodized epitaxial Graphene with a large number of Oxygen related defects has been reported which has detected all the DNA bases that are well resolved on the DPV. The detection limit for dsDNA has been reported 1 μgmL−1. The anodized epitaxial Graphene can be used to differentiate between the dsDNA and ssDNA and is highly selective.365 The single nucleotide mismatch of DNA by the hybridization can be detected by the anodized epitaxial Graphene on the DPV. The covalent grafting of the DNA sensor on anodized epitaxial Graphene provides higher sensitive and selective response in comparison to pi-stacked DNA sensor.366 The Graphene based sensors have been employed to detect a wide range of DNA molecules.367,368
The fluorescence resonance energy transfer based process has been used in GO based sensor for the detection of DNA. The detection is highly selective to even a single mismatch in DNA and the limit of detection has been reported to be 40 pM.367 The additional surface area provided by the presence of nanoparticles, enhanced electro affinity for the analyte or improved redox properties has been provided by the Graphene in the presence of substances like gold nanoparticles,369 Fe3O4,370 nickel hydroxide,371 and cobalt pthalocyanine.372
Detection of other biomolecules
The advanced electrochemical detection of dopamine by the Graphene based sensors has been investigated extensively and reported.373–378 The Fe3O4-NH2 in Graphene has been used to detect ascorbic acid, dopamine, and uric acid which have exhibited enhanced electrochemical oxidation of these acids as compared to the Graphene electrodes.370 The dopamine-graphene-chitosan complex has been synthesized and electrodeposited on a GCE in a molecularly imprinted polymer electrochemical sensor in which the lower limit of detection has been reported to be 10 pM.379
The Graphene has been used to detect the uric acid,370,371 ascorbic acid,370,371 L-cysteine,372 glutamate380,381 and dopamine.369–371,382–384 The Graphene nanosheets have been used as immunodetectors for the detection of carcinoembryonic antigen and alpha-fetoprotein in the biological fluids. The simultaneous monitoring of both the antigens has been reported with the wide working ranges from 0.01–200 ngmL−1 and 0.01–80 ngmL−1 for the carcinoembryonic antigen and alpha-fetoprotein respectively with a limit of detection 1 pgmL−1 for both of them.385 A reduced GO based electrochemical immunosensor for the detection of the antigen in Enzyme Linked Immuno Sorbent Assay (ELISA) test has been reported by employing a rGO, poly(benzene-ringpoly(ethyleneglycol)methacrylate-N-acryloxy succinimide)(poly(B.P.N.)layer) and bovine serum albumin (B.S.A.). The electrochemical immunosensor displayed a limit of detection 100 fgmL−1 for the detection of antigen mouse IgG.386 In another research, the Micro-cystine LR-aptamers are attached onto the Graphene surface to fabricate an aptasensor system. The sensor exhibited a wide linear range of 1–100 pM and a low limit of detection of 0.8 pM to detect the aptamer.387 An electrochemical aptasensor employing rGO has been fabricated for the evaluation of dynamic cell surface of N-glycan in which the concanavalin A (Con A) has been attached on the surface of dendrimer- conjugated rGO modified GCE which causes multivalent bonding between Con A and N-glycan on the surface of cell as shown in the Fig. 10. As a result of which the efficiency of aptasensor for the cell capture has been enhanced.388
Figure 10. Synthesis of an electrochemical aptasensor employing rGO for the evaluation of dynamic cell surface of N-glycan, Reprinted with permission from388 (Copyright 2014, American Chemical Society).
Download figure:
Standard image High-resolution imageCarbon Nanohorn Based Electrochemical Biosensor
The single walled carbon nanohorn (SWCNH) based biosensors employing attachment of enzymes or proteins have been vastly investigated and reported.388–392 The enzymes or proteins have been attached to the surface of SWCNHs. The Glucose oxidase has been attached in the Nafion-SWCNH composite which has been used for maintaining the enzymatic activity in the glucose biosensor. The sensor has exhibited a lower limit of detection, high sensitivity and high selectivity to the detection of glucose.388 An electrochemical biosensor has been reported by attaching protein myoglobin on the surface of SWCNHs which has been functionalized by the non-covalent bonding of poly (styrenesulphonate). The biosensor exhibited good electro catalysis to the reduction of H2O2.390 The CNH based immunosensor has been developed for the detection of microcystin-LR released by the cyan bacterial bloom which is a highly toxic species. The surface area of CNHs has been used to restrict the recognition components. The carboxylated CNHs are deposited at a GCE surface where they bioconjugate to microcysin-LR. The electrode platform and free MC-LR have a tendency to bind to the horseradish peroxidase-labeled (MC-LR) antibody. The ratio of concentration of CNH labeled MC-LR and solution MC-LR are correlated by the current detected for the enzymatic reaction. The biosensor has reported a good response for the MC-LR with a limit of detection 0.3 μgl−1 which is less than the safe limit of 1μgL−1 as per the recommendations of WHO in the drinking water.393
A highly sensitive impediametric immunosensor for the detection of alpha-fetoprotein has been developed and reported that employs the electro catalytic activity of CNHs coupled with an enzymatic biocatlytic precipitation process. The two bioenzymes which are horseradish peroxidase and glucose oxidase has been used to fabricate the biosensor. A limit of detection of 0.33 pg ml−1 has been reported for the detection of alpha-fetoprotein by employing this CNH based immunosensor with high selectivity.394 The alpha-fetoprotein has also been detected by the use of a multiplexed CNH sensor containing Au nanoparticles and streptadivin. The Au nanoparticles are in-situ grown on the carboxylated CNHs which are further functionalized with streptadivin. The sensor exhibited a limit of detection 0.024 pg ml−1 to the detection of alpha-fetoproteins. The carcinoembryonic antigen has also been detected by employing this CNH immunosensor for which limit of detection 0.032 pg ml−1 has been reported.395
The CNH has the ability to quench the fluorescence of an analyte.396 They have been used as an optical biosensor for the detection of DNA. The oligonucleotide sequence related to the human immunodeficiency virus (HIV) has been detected by making use of the capability of CNHs to quench the fluorescence. The CNHs has different binding affinity for ss-DNA and ds-DNA. The HIV virus has a strong affinity for binding on the surface of CNH through pi-pi interactions. As a consequence of it, the quenching of fluorescence occurs. The binding affinity is eliminated if a double strand is obtained in the presence of the target sequence.397
The functionalization of CNHs with a fluorescin labeled protein by non-covalent bonding has been employed to detect the presence of thrombin. The CNHs have the capability to quench the fluorescence. The dye labeled peptide gets chopped off in the presence of thrombin protease. With the release of peptide, the fluorescence is again recovered back. The sensor displayed a limit of detection of 100 pM to the detection of thrombin. The high selectivity of the biosensor has been demonstrated by employing 100 nM human IgG and 100 nM bovine serum albumin solutions.398
Carbon Dots Based Electrochemical Biosensors
The CDs exhibit a biocompatibility that can be engineered for the development of invivo biosensors. The CD based biosensors present a favorable environment for the detection of ions which are biologically very significant. The CD based nano sensor functionalized with zinc responsive quinoline derivatives has been reported for the detection of quinoline. As a consequence of binding of Zn2+ ions on the nano surface of CDs, there is a shift in the fluorescence from weak blue to the strong green color. The CD based biosensor displayed a limit of detection of 6.4 nM to the detection of quinoline molecules which is much lower than the detection limit of 270 nM investigated for the free quinoline molecules.399
The CDs have been employed to detect a vast number of biomolecules like dopamine,400 DNA,401,402 cholesterol,403 glucose,404,405 L-cysteine,406 ovalbumin,407 cholines,408 ascorbic acid,409,410 histene,411 human carcino embryonic antigen.412
The biosensors employing GQDs and CQDs to detect biomolecules have been reviewed and reported.413 The detection of hemoglobin has been reported by using a fluorescent nano biosensor which is based on the molecularly imprinted polymers and CQDs by means of the fluorescence quenching. The fabricated biosensor exhibited a linear range of 0.77–7.7 nM and a limit of detection 0.77 nM for the detection of hemoglobin with excellent sensitivity and high selectivity. The employment of molecularly imprinted polymers and CQDs has reported an increased selectivity to hemoglobin.414
Another electrochemical immune sensor has been reported to detect cardiac troponin I by employing GQDs.The1-ethyl-3-(3-dimethylaminopropyl) carbodiimide/N-hydrogysuccinimide (EDC/NHS) has been used for the covalent coupling to the GQDs on an amino functionalized Au-printed electrode. The EDC coupling has been employed for depositing 4-aminothiophenol (4-APT) and poly (amidoamine) (PAMAM) dendrimer on the GQDs. The detector exhibited a limit of detection of 20 fgmL−1 and 25 fgmL−1 for DPV and CV techniques respectively for the detection of troponin I.381 The various steps involved in the detection of CTnI involving the use of an Au/GQD/PAMAM nanohybrid electrode are shown in the Fig. 11.415
Figure 11. Various steps involved in the detection of CTnI involving the use of an Au/GQD/PAMAM nanohybrid electrode, Reprinted with permission from.415
Download figure:
Standard image High-resolution imageCarbon Nanofibres Based Electrochemical Biosensors
The CNFs can be used as a substrate for the attachment of biomolecules for electrochemical detection purposes. The surface functional groups can be introduced into CNFs by the chemical treatment that not only enhances their solubility but also increases their selective interaction with biomolecules. The oxygen containing surface functional groups can be increased in them by their oxidation with conc. acids.416 The CNFS can also be oxidized by nitric acid (HNO3), Hydrogen Peroxide (H2O2), potassium permanganate (KMnO4), Ruthenium tetra oxide (RuO4) and Potassium Hydroxide (KOH).417–419 With the formation of functional groups on the surface of CNFs, it becomes easier for the attachment of selective biomolecules such as proteins, enzymes and DNA on their surface. The CNFs do possess more functionalized surface area for attachment of biomolecules in comparison to CNTs.420
Detection of glucose
A critical review of CNFs based biosensors has been reported.421 The Ni nanoparticles loaded CNFs prepared by electro spinning have been used to detect the presence of glucose. The fabricated biosensor exhibited a limit of detection 1 μM with a linear range of 2–2500 μM.422 In another study, the bimetallic CuCo nanoparticles embedded in CNFs by employing electro spinning has been used to detect the presence of glucose in human urine. The fabricated CuCo-CNFs electrode has exhibited a limit of detection 1 μM with a linear range of 0.02–11 mM.423 The Pt nanoparticles loaded CNFs have exhibited a linear range of 2–20 mM to the detection of glucose.424 The presence of glucose has also been detected by Copper oxide nanoneedles with reduced graphene oxide with CNFs. The fabricated composite has exhibited a limit of detection 0.1 μMin the linear range of 1–5.3 mM.425 Another research has reported a limit of detection 0.03mM in the linear range of 0.06–6 mM to the detection of glucose for a palladium nanoparticles decorated helical CNF hybrid.426
Detection of other biomolecules
The palladium nanoparticles loaded CNFs have been used to prepare an electrode which has been used for the detection of dopamine, uric acid and ascorbic acid. The fabricated biosensor has reported a limit of detection of 0.2 μM, 0.7 μM and 15 μM in the linear range of 0.5–160 μM, 2–200 mM and 4 mM for the detection of dopamine, uric acid and ascorbic acid respectively.427 In another study, the GCE modified with CNFs loaded with Ag-Pt alloy nanoparticles have exhibited the detection of dopamine in the presence of uric acid and ascorbic acid with a limit of detection of 0.11 μM with a linear range of 10–500 μM.428 The magnetic composite of polydopamine,laccase and Ni nanoparticles decorated CNFs prepared by electro spinning and high temperature carbonization method have exhibited a limit of detection of 0.69 μM for catechol in the real water samples in a linear range from 1 to 9.1 mM along with good selectivity.429
The vertically aligned CNFs i.e. CNFs grown perpendicular to the substrate is also the potential candidate for use in electrochemical biosensing applications. A nanoelectrode array based on vertically aligned CNFs have been reported for the detection of C-reactive protein with a limit of detection of 90 pM.430 The molybdenum disulphide nanosheet arrays with CNFs have been synthesized by electro spinning and hydrothermal synthesis and are employed as an electrode for the detection of Levodopa and uric acid by using cyclic voltammetry and differential pulse voltammetry.Alimit of detection of 0.91 and 0.73 μAμM−1 to the detection of Levodopa and uric acid respectively in the linear range of 1–60 μM has been reported.431
Detection of DNA hybridization/proteins
The Vertically aligned CNF arrays grown on silicon wafers has been reported to detect the DNA hybridization by employing AC Voltammetry. The oligonucleotide probe molecules functionalized CNFs hybridize with target DNA strands causing DNA hybrids due to the replacement of guanine bases from the oligonucleotide probes with Inosine groups. The signal of guanine oxidation is amplified by the presence of electrochemical mediator Ru(bpy)23+ in the solution. The study of scans of sequential AC Voltammetry detects the presence of hybridized DNA with a high sensitivity of approx.1000 PCR amplicon molecules with excellent selectivity.432 Another electrochemical biosensor have been prepared by attaching an anchoring layer of chitosan, carboxyl group functionalized CNFs and glutaraldehyde on a GCE to detect the DNA hybridization. By using cyclic voltammetry, the hybridization is detected by the change in oxidation peak current of ferrocene carboxylic acid at the sensor interface. The sensor exhibited a limit of detection of 88pM in the dynamic range of 0.50 and 40nM with an excellent selectivity by exhibiting insignificant current change for non-complementary DNA targets.433
The CNFs modified onto carbon paste electrode has been used for the detection of amino acids L-tryptophan-tyrosine and L-Cysteine with a limit of detection 0.1 μM.434 For the early diagnosis of myocardial infection, a CNF based nanoelectrode array for cardiac troponin-I have been reported which exhibited a limit of detection 0.2 ng ml−1 to the detection of cardiac troponin-I within a linear range of 0.25–1.0 and 5.0–100 mg ml−1.435
The wild M13 bacteriophage particles have got the potential to modify the features of electrodes as a consequence of non-specific electrostatic interactions and pi-pi interactions between them and CNFs. This results into uniform distribution of CNFonto electrode surface. The cyclic voltammograms of GCE electrodes with M13 particles along with CNFs have demonstrated an improvement in their electrochemical properties. The addition of bacteriophage particles has a direct influence on the aggregation of CNFs as it prevents their aggregation. Also, with the addition of bacteriophage particles into CNFbased electrodes, the electro active surface is well produced for the electrochemical biosensing applications. The cyclic voltammetry results of electro catalytic response of oxidation of L-Cysteine have exhibited an improvement if M13 bacteriophage particles are incorporated into the CNF based electrodes.436
Carbon Black Based Electrochemical Biosensors
Detection of glucose
The application of Carbon black (CB) based nanomaterials for the electrochemical biosensing have been widely reported. The Carbon black has been used for the electrochemical sensing of biomolecules. The Carbon black based sensors has been used for the detection of glucose. A biosensor employing poly (Sodium 4-styrenesulphonate) attached on the CB surface has been reported that can be used for the detection of glucose.437 The glucose oxidase biosensors based on CB nanoparticles in electrode have exhibited a better electron transfer between the glucose oxidase enzyme and the CB electrode in comparison to other electrodes made up of SWCNTs, MWCNTs, C60, GO, rGO and SWCNHs. They have exhibited comparable limit of detection, linear range and better sensitivity.438 A non-enzymatic biosensor has been reported employing carbon nanoparticles functionalized carbon black composite modified GCE fabricated by microwave method to detect the presence of glucose in biological fluids and human serum samples.439
Detection of DNA hybridization
The CB has also been used for the biosensing of DNA molecules. A Tungsten disulphide –acetylene black composite formed by using hydrothermal process has been reported for the DNA biosensing applications. The fabricated nanocomposite with GCE electrode with Au nanoparticles has exhibited a limit of detection of 1.2 × 10−16molL−1 in the range from 1.0 × 10−15molL−1 to 1.0 × 10−10molL−1to the target ssDNA.440 Another biosensor has been fabricated by using composite formed by CuS nanosheets and acetylene black(AB) by employing solvothermal process with the help of ethylene glycol. The surface of GCE was modified with CuS-AB film,later gold nanoparticles are deposited on it by the electro deposition method. The detector exhibited a decrease in current for the [Fe (CN)6]3-redox couple every time when there is a hybridization between the attached ssDNA probe and target ssDNA.441 In another significant research, an electrochemical biosensor employing pencil graphite electrodes modified with CB has reported the detection of micro RNA-125a hybridization in samples by using impedance spectroscopy. The biosensor exhibited a limit of detection of 10pM with a linear range of 0.008–15 μgml−1 with good selectivity. The fabricated biosensor has been used for the detection of micro RNA-125a in real serum samples proving its viability for diagnostic purposes.442
Detection of proteins
The presence of oxygenated functional groups and active sites on the CB particle surface facilitates the biosensing process. A biosensor employing GCE substrate has been modified with a mixture of hemoglobin-Carbon black (Hb-CB) which is anchored by using Nafion film. By using cyclic voltametry, it has been confirmed that there is a direct electron transfer from the Hb protein to the CB modified electrode which is attributed to the presence of oxygenated functional groups and active sites on the CB particle surface.443 The direct electron transfer of different Fe(III) hemeproteins attached on the electrodes modified with CB and didodecycldimethyl ammonium bromide have been thoroughly investigated and reported.444 The direct electron transfer of Cytochrome C and Hb biosensor based on CB nanoparticles have also been reported.445,446
Enzymatic biosensors
The Carbon black has the potential to substitute graphite powder for the fabrication of enzyme linked biosensors. The enzymatic biosensor constructed by combining carbon black paste with tyrosinase enzyme have demonstrated highest sensitivity of 625nAlμM with a limit of detection of 0.008 μM to the detection of catechol, thereby, showing electrochemical features even better than graphite and carbon nanotube tyrosinase paste electrode.447 The Bisphenol A has been used in the coating of metallic cans for storage of tomato juice samples. Its detection is very important as it can cause serious health implications. A biosensor based on laccase-thionine-carbon black –modified Screen printed electrode has been reported to detect the presence of Bisphenol A employing the use of nanostructured carbon black coupled with electrochemical mediator thionine. The fabricated biosensor has demonstrated a sensitivity of approx. 5.0nA μm−1 and limit of detection 0.2 μM.448 Another biosensor capable of detecting H2O2 in the samples of oxygenated water and milk has been reported in which Zein, a protein in corn seed is combined with carbon black to attach the haemoglobin. The fabricated biosensor has demonstrated a limit of detection 4 × 10−6molL−1 using differential pulse voltammetry.449 The urease enzyme attached to CB paste electrode modified with a flexible membrane based on terylene with polyvinyl alcohol has been used to detect the presence of urea in milk samples. The presence of urea was detected by the amperometric sensing of carbamic acid which is produced during the enzymatic conversion of urea. The biosensor exhibited a linear response in the range 2.0 × 10−4molL−1 to 4.0 × 10−3molL−1.450
An enzymatic biosensor to detect the presence of hydroquinone and Catechol has been reported in which quatnernized cellulose nanoparticles with CB and Au nanoparticles composite is used for the attachment of hemocyanin enzyme. The fabricated biosensor has exhibited a limit of detection of 8.0 × 10−9molL−1 and 8.7 × 10−7molL−1 in the linear range from 4.5 × 10−7molL−1to 2.4 × 10−5molL−1 and 3.7 × 10−7molL−1 to 5.0 × 10−5molL−1 for hydroquinone and catechol respectively.451
Non-enzymatic biosensor
Recently, a non-enzymatic biosensor PdCu/Carbon Black based biosensor has been reported that has detected the presence of H2O2 released from living cells in real time. The fabricated biosensor exhibited a limit of detection of 54nM with a wide detection range from 0.4 μM to 5.0mM.452 Figure 12 shows the TEM image of PdCu particles dispersed on Carbon Black, High Resolution TEM image of PdCu alloy, Histogram graph of PdCu nanoparticles size distribution and TEM image of Carbon Black in the fabricated biosensor.452
Figure 12. (A) TEM image of PdCu particles dispersed on Carbon Black (B) High Resolution TEM image of PdCu alloy (C) Histogram graph of PdCu nanoparticles size distribution (D) TEM image of Carbon Black, Reprinted with permission from.452
Download figure:
Standard image High-resolution imageDetection of pesticides
The CB based sensors has also been used for detecting the presence of pesticides in the samples.
The acetyl cholinesterase (AChE) biosensor, using unsubstituted pillar5arene as electron mediator, has been fabricated for the detection of organophosphate and carbamate pesticides in the samples of peanut and beetroot. The AChE is attached on the surface of CB placed on GCE by carboimide binding. The fabricated biosensor detected malaoxon,paraoxon,carbofuran and aldicarb with a limit of detection 4 × 10−6, 5 × 10−9, 2 × 10−10 and 6 × 10−10 M in the linear range of 1 × 10−11- 1 × 10−6M, 1 × 10−8- 7 × 10−6M, 1 × 10−10- 2 × 10−6M and 7 × 10−9- 1 × 10−5M respectively.453
A screen printed electrochemical electrode has been reported for the detection of paraoxon by using enzyme butyrylcholinesterase (BChE). The CB nanoparticles are dispersed on the surface of electrode in a dimethylformamide water mixture. The BChE was attached on the surface of electrode by means of cross-linking. The fabricated biosensor exhibited a limit of detection 5 μgl−1 for a concentration of paraoxon up to 30 μgl−1 and stability up to 78 days of storage at room temperature in waste water samples.454
Conclusions
The excellent electrochemical properties of CNMs have paved the way for their use in a wide range of electrochemical biosensors for the detection of biomolecules and for various biomedical applications. The electrochemical sensing performance (sensitivity and selectivity) of the CNTs can be modified by the covalent or non-covalent functionalization of CNTS. In order to enhance their sensitivity and selectivity, a mixture or hybrid or composite of these nanomaterials with organic or inorganic compounds has to be used. The CNTs have exceptional properties to enhance the electrochemical reaction of the biomolecules. The CNT modified electrodes can act as linking agents to the biomolecules. They do have large surface area that makes them suitable for the attachment of substrate on their surface. The CNTs with non-covalent functionalization has been effective for the attachment of biomolecules with more biocompatibility. The electrochemical characterization of the nanomaterials can be done by employing various electrochemical techniques. The amperometric bio-sensing of the substrates has been feasible due to the electro catalytic activity of CNT based electrodes. The CNTs have been used for the detection of biomolecules with immense applications in the biomedical field. Graphene and GO nano-sheets have been engineered for the detection of biomolecules and as efficient nano carriers for the drug delivery systems without any use of catalysts. The Graphene electrode has exhibited a better performance for the detection of dopamine, serotonin ascorbic acid, uric acid, DNA and glucose etc.
Graphene, being non toxic in nature, is more suitable for use in bio-sensing applications than CNTs due to its large surface area for the attachment of biomolecules and more uniform distribution of active electron sites. The electrochemical biosensing features like sensitivity and selectivity can be enhanced by customizing the properties of Graphene by various methods.
Other allotropic modification of the Carbon, The SWCNH based electrochemical biosensors have been vastly investigated and reported. The sensor has exhibited a lower limit of detection, high sensitivity and high selectivity to the detection of glucose, H2O2, microcystin-LR released by the cyan bacterial bloom, alpha-fetoprotein carcinoembryonic, DNA and Thrombin etc.
The CDs, CQDs and GQDs have exhibited better efficiency and selectivity for the attachment of proteins, enzymes and antibodies by adsorption, covalent deposition or chemical reactions. These materials have exhibited chemical stability, solubility in water and organic solvents. They have displayed excellent electro catalytic display towards the detection of H2O2 and O2. The CDs have been employed to detect a vast number of biomolecules like dopamine, DNA, cholesterol, glucose, L-cysteine, ovalbumin, cholines, ascorbic acid, histene and human carcino embryonic antigen,quinoline,Hemoglobin and cardiac troponin I etc. with high sensitivity and high selectivity.
In Carbon Black, the presence of sp2 hybridized carbon atom edge planes and oxygenated groups over the nanomaterials make them capable to attach biomolecules on their surface to act as electrochemical biosensors. They can be used for the detection of glucose, DNA hybridization, uric acid, dopamine, pesticides etc. for the biosensing applications. The Carbon Nano Fibres do possess attributes like good electrical conductivity, large surface area, biocompatibility and easy fabrication process that are vital for electrochemical sensing applications. The CNFs can be used to detect glucose, DNA hybridization, uric acid, dopamine, proteins etc.
Challenges/Future Work/Opportunities
- 1.To devise new methods for the more comprehensive characterization of materials in order to study the structure, quality and properties of CNTs within the metal oxide tube.
- 2.To develop techniques for the separation of CNTs from the impurities which need harsh treatment for their removal this can damage the CNTs resulting into their shortened length.
- 3.To create and develop new methods for the separation of metallic CNTs from the semiconducting CNTs.
- 4.To reduce the formation of CNT bundles that can occur naturally due to the presence of vandarwaals attractions.
- 5.To tackle the difficulties in obtaining small metal catalyst nanoparticles, their equal dispersion on the substrate and to prevent their aggregation.
- 6.To develop technology for the formation of well defined number of walls in CNTs.
- 7.To promote more scientific research and study in the field of CNMs with respect to their physical and chemical properties as they are less well defined in comparison to other competing materials.
- 8.To develop techniques in order to obtain pure grapheme nanosheets.
- 9.To develop techniques to fabricate biosensors with lower limit of detection for a wide range of biomolecules.
- 10.To develop technology to fabricate biosensors with the characteristic features to detect all the diseases causing agents.
- 11.To develop methods for enhancing and tuning the features of CNTs for further improvement in the device characteristics of biosensors.
- 12.To devise the methods for tailoring the density of states and structure of bonds in CNMs for tailoring their intrinsic properties for better electron transport between the electrode and target or analytes.
- 13.To develop effective micro structural characterization techniques as the small variation in the structure of CNMs can cause a large change in their electrochemical behavior.
- 14.To develop techniques to enhance the small detection range of biosensors due to which the delay in detection, treatment and management of the disease may be avoided.
- 15.Since the CNMs based biosensors are unable to differentiate between the responses of compounds with same functional group in their structures, their accurate quantification becomes difficult. The addition of some other materials such as metals, metal oxides, organometallic.