Abstract
The solid electrode interphase (SEI) on graphite electrodes is important to the performance, calendar life, and safety characteristics of lithium-ion cells. This article examines the SEI formed on binder-free graphite electrodes prepared by electrophoretic deposition. X-ray photoelectron spectroscopy, Fourier transform infrared spectroscopy, and thermogravimetric analysis data were obtained on electrodes cycled in cells containing four electrolytes comprising ethylene carbonate: ethylmethyl carbonate (3:7 by weight) solvent and 
 ,
, 
 ,
, 
 , or
, or 
 salt. Our observations suggest that, in addition to solvent reduction, the reduction of electrolyte salts plays an important role in SEI formation. Mechanisms to account for the formation of these SEI constituents are included in the article.
salt. Our observations suggest that, in addition to solvent reduction, the reduction of electrolyte salts plays an important role in SEI formation. Mechanisms to account for the formation of these SEI constituents are included in the article.
Export citation and abstract BibTeX RIS
Graphite is widely used as the anode material in commercial lithium-ion batteries. It is well known that the electrolyte components react with the anode surface to form a passivation film, the solid electrolyte interphase (SEI) during initial charging of a lithium-ion battery.1 The SEI layer protects the graphite during subsequent cycling; the composition, morphology, and stability of the SEI are known to critically affect cycling performance and storage life of a lithium-ion cell.2–6
However, there is a continuing debate on the formation mechanisms and composition of the graphite SEI. Various electrolytes have been used in lithium-ion batteries, and the morphology and chemical composition of the SEI are likely dependent on the characteristics of the electrolyte (solvent, lithium salt, and additives).5, 7–15 The standard electrolyte used in commercial lithium-ion cells is a solution of  salt in a mixture of carbonate solvents, including ethylene carbonate (EC) and dialkyl carbonates, such as ethyl methyl carbonate (EMC). Although
salt in a mixture of carbonate solvents, including ethylene carbonate (EC) and dialkyl carbonates, such as ethyl methyl carbonate (EMC). Although  -based electrolytes have the best combination of properties, other electrolytes have been considered because of their unique properties. For instance,
-based electrolytes have the best combination of properties, other electrolytes have been considered because of their unique properties. For instance,  -based electrolytes demonstrate good thermal stability at elevated temperature, but their conductivities are lower than those of
-based electrolytes demonstrate good thermal stability at elevated temperature, but their conductivities are lower than those of  -based electrolytes.14 Electrolytes based on the recently developed lithium salts
-based electrolytes.14 Electrolytes based on the recently developed lithium salts  (LiBOB) and
(LiBOB) and  demonstrate excellent ionic conductivity and thermal stability, but their low-temperature performance has been questioned.16–20
demonstrate excellent ionic conductivity and thermal stability, but their low-temperature performance has been questioned.16–20
A recent investigation of electrode impedance in cells containing  -based composite positive electrodes, mesocarbon microbead (MCMB)-10 graphite-based composite negative electrodes, and electrolytes with 3EC:7EMC solvent and
-based composite positive electrodes, mesocarbon microbead (MCMB)-10 graphite-based composite negative electrodes, and electrolytes with 3EC:7EMC solvent and  ,
,  , LiBOB, and
, LiBOB, and  salts showed that the electrolyte salt has a strong influence on the impedance of graphite electrodes measured after initial cycling.21 The graphite electrode showed the following impedance trend with salt composition:
salts showed that the electrolyte salt has a strong influence on the impedance of graphite electrodes measured after initial cycling.21 The graphite electrode showed the following impedance trend with salt composition:  ; that is, the electrode displayed the highest and lowest impedances in cells containing LiBOB and
; that is, the electrode displayed the highest and lowest impedances in cells containing LiBOB and  , respectively. The study concluded that the nature of the graphite SEI formed in the various electrolytes was an important determinant of the electrode impedance.21 Because electrolyte salt was the only variant, the study postulated that the electrolyte salt has a strong effect on the composition and morphology of the graphite SEI, which is in accordance with the conclusions of other researchers.22–29
, respectively. The study concluded that the nature of the graphite SEI formed in the various electrolytes was an important determinant of the electrode impedance.21 Because electrolyte salt was the only variant, the study postulated that the electrolyte salt has a strong effect on the composition and morphology of the graphite SEI, which is in accordance with the conclusions of other researchers.22–29
Detailed and conclusive analysis of the anode SEI is complicated by the presence of inactive components, such as the polyvinylidene difluoride (PVdF) binder, in conventional composite electrodes. These components often obscure spectral information arising from chemical species in the SEI layer. To alleviate this problem, we developed a binder-free (BF) electrode for the study of graphite SEI layers. Initial data from these electrodes, including materials characterization and electrochemical performance, were reported earlier.15 In this article, we describe the study of SEI layers formed on BF graphite electrodes cycled in cells containing four electrolytes: 
 ,
, 
 ,
, 
 , and
, and  LiBOB in EC:EMC solvent (3:7 by weight). The
LiBOB in EC:EMC solvent (3:7 by weight). The 
 electrolyte was from Tomiyama Chemical Industries (Japan). The high-purity 3EC:7EMC solvent used to prepare the other electrolytes was obtained from Ferro (USA). High-purity
electrolyte was from Tomiyama Chemical Industries (Japan). The high-purity 3EC:7EMC solvent used to prepare the other electrolytes was obtained from Ferro (USA). High-purity  and
and  salts were acquired from LithChem International and Central Glass Co. (Japan), respectively. The LiBOB salt was obtained from Chemetall GmbH (Germany); the salt was further purified at Argonne by a dissolution∕filtration procedure before electrolyte preparation. The BF graphite electrode samples were examined by Fourier transform infrared spectroscopy (FTIR) with attenuated total reflection (ATR), X-ray photoelectron spectroscopy (XPS), and thermogravimetric analysis (TGA). SEI formation mechanisms were formulated based on these data and are included in this article.
salts were acquired from LithChem International and Central Glass Co. (Japan), respectively. The LiBOB salt was obtained from Chemetall GmbH (Germany); the salt was further purified at Argonne by a dissolution∕filtration procedure before electrolyte preparation. The BF graphite electrode samples were examined by Fourier transform infrared spectroscopy (FTIR) with attenuated total reflection (ATR), X-ray photoelectron spectroscopy (XPS), and thermogravimetric analysis (TGA). SEI formation mechanisms were formulated based on these data and are included in this article.
Experimental
The BF graphite electrodes were prepared by electrophoretic deposition (EPD).15, 30 In EPD, colloidal particles suspended in a liquid medium migrate under the influence of an electric field and are deposited onto an electrode. The EPD solution was prepared by dispersing graphite particles (SFG-6, TIMCAL,  ) in anhydrous acetonitrile by ultrasonication. Small amounts
) in anhydrous acetonitrile by ultrasonication. Small amounts  of triethylamine were added to the EPD bath, to form surface charges on the graphite particles. Copper and stainless steel foils
of triethylamine were added to the EPD bath, to form surface charges on the graphite particles. Copper and stainless steel foils  were used as cathode and anode, respectively. The graphite particles were deposited on the copper foil by applying a dc voltage of
were used as cathode and anode, respectively. The graphite particles were deposited on the copper foil by applying a dc voltage of  for
for  to the electrodes. The graphite electrode, thus prepared, was dried in a vacuum oven at
to the electrodes. The graphite electrode, thus prepared, was dried in a vacuum oven at  to completely remove the residual acetonitrile and triethylamine. The average graphite loading density of the resulting electrode was
to completely remove the residual acetonitrile and triethylamine. The average graphite loading density of the resulting electrode was  .
.
Cell assembly, galvanostatic cycling, and cell disassembly were conducted in an Ar-atmosphere glove box (
 ,
, 
 ). Electrochemical cycling was conducted using 2032-type coin cells containing small pieces
). Electrochemical cycling was conducted using 2032-type coin cells containing small pieces  of the BF graphite electrode, metallic lithium counter electrode, and Celgard 2325 (
of the BF graphite electrode, metallic lithium counter electrode, and Celgard 2325 ( thick) separator. The coin cells were cycled three times between 2.0 and
thick) separator. The coin cells were cycled three times between 2.0 and  at room temperature with a current density of
at room temperature with a current density of  . The cells were then disassembled, and the delithiated graphite electrodes harvested for SEI examination. The cycled electrodes were examined both "as is" ("unrinsed") and after EMC rinsing ("rinsed") to wash off excess electrolyte residue. The samples were rinsed by soaking in
. The cells were then disassembled, and the delithiated graphite electrodes harvested for SEI examination. The cycled electrodes were examined both "as is" ("unrinsed") and after EMC rinsing ("rinsed") to wash off excess electrolyte residue. The samples were rinsed by soaking in  EMC for
EMC for  , after which the EMC was removed by decanting. Fresh BF graphite samples were "dipped" in the various electrolytes and examined to identify characteristic features of the electrolyte residue. All electrodes were vacuum dried overnight at room temperature before examination.
, after which the EMC was removed by decanting. Fresh BF graphite samples were "dipped" in the various electrolytes and examined to identify characteristic features of the electrolyte residue. All electrodes were vacuum dried overnight at room temperature before examination.
FTIR data provide information on functional groups present in the top  of the graphite electrode, without destroying the surface species. Our FTIR measurements were conducted on a Thermo Nicolet IR 300 spectrometer stored in a glove bag that was purged with high-purity Ar (i.e., the data were collected without any sample air exposure). The spectra were acquired in the ATR mode with
of the graphite electrode, without destroying the surface species. Our FTIR measurements were conducted on a Thermo Nicolet IR 300 spectrometer stored in a glove bag that was purged with high-purity Ar (i.e., the data were collected without any sample air exposure). The spectra were acquired in the ATR mode with  resolution and 128 total scans. The measurements were conducted at three different spots on the sample to avoid possible error and to examine uniformity of the SEI layer.
resolution and 128 total scans. The measurements were conducted at three different spots on the sample to avoid possible error and to examine uniformity of the SEI layer.
After FTIR-ATR, the samples were examined by XPS, which provides information on chemical species present in a  thick layer on the electrode surface. Each sample was exposed to air for a few seconds during insertion into the XPS analysis chamber. The XPS spectra were obtained on a PHI 5500 system using Al
thick layer on the electrode surface. Each sample was exposed to air for a few seconds during insertion into the XPS analysis chamber. The XPS spectra were obtained on a PHI 5500 system using Al  radiation
radiation  under ultrahigh vacuum conditions; the data were acquired at two different spots for each sample. The C 1s, O 1s, F 1s, P 2p, and B 1s spectra were calibrated based on the C 1s graphite peak binding energy at
under ultrahigh vacuum conditions; the data were acquired at two different spots for each sample. The C 1s, O 1s, F 1s, P 2p, and B 1s spectra were calibrated based on the C 1s graphite peak binding energy at  or based on the hydrocarbon peak at
or based on the hydrocarbon peak at  . Element concentrations were calculated using integrated peak intensities and atomic sensitivity factors from the PHI instrumentation software.
. Element concentrations were calculated using integrated peak intensities and atomic sensitivity factors from the PHI instrumentation software.
TGA measurements were conducted on rinsed graphite electrode samples to obtain information on volatile organic and inorganic compounds in the SEI. The graphite was removed from the copper current collector in an Ar-filled glove box. The TGA data were collected on a TA Instruments SDT 2900 by ramping the temperature from room temperature to  at a rate of
at a rate of  . The samples were exposed to air prior to and during the TGA measurements.
. The samples were exposed to air prior to and during the TGA measurements.
Results
Electrochemical cycling
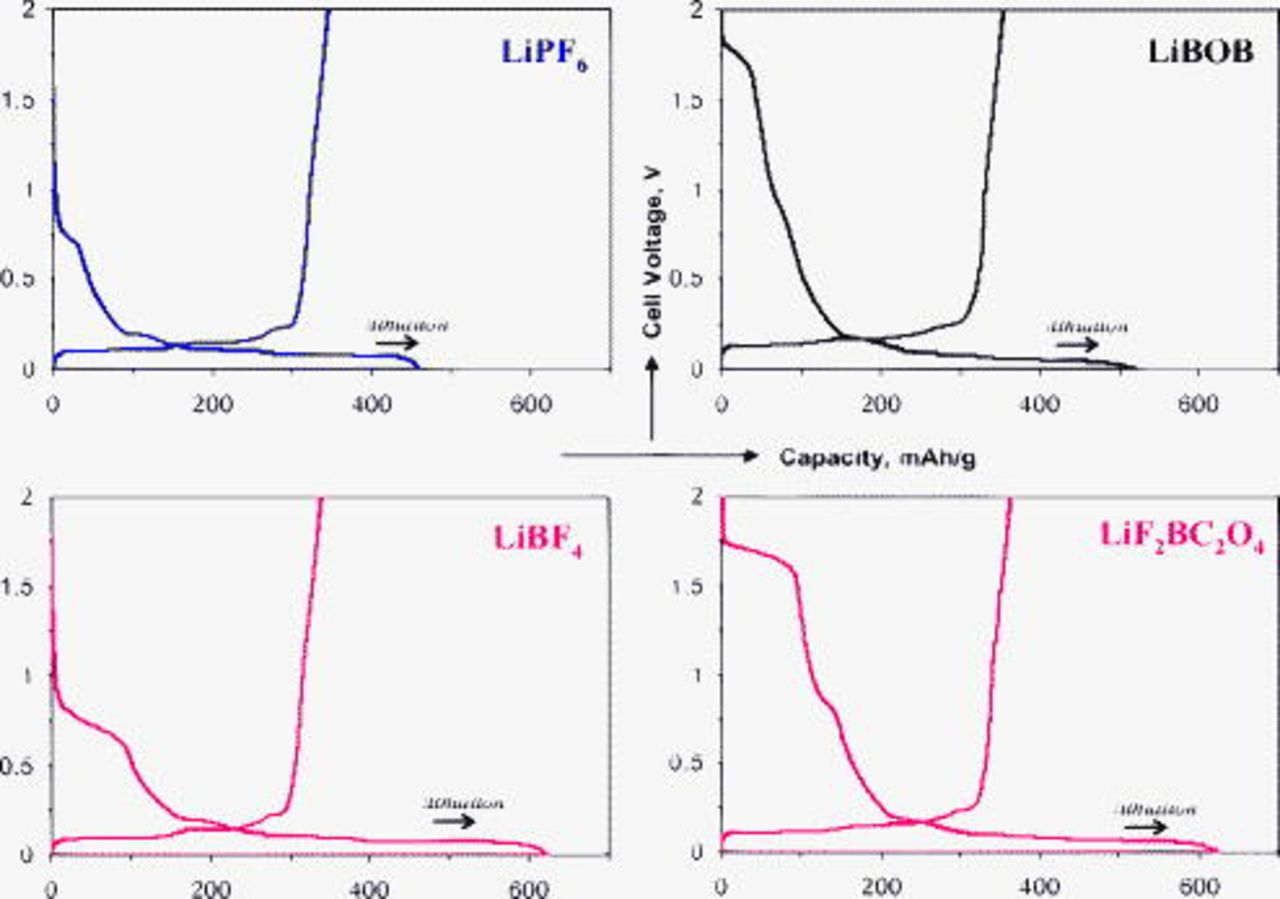
Figure 1 displays the first lithiation–delithation cycle of the BF graphite electrode cells containing various electrolytes; corresponding differential capacity plots are shown in Fig. 2. During the first lithiation, the LiBOB and  cells show a long voltage plateau at voltages of
cells show a long voltage plateau at voltages of  , which are not observed for the
, which are not observed for the  and
and  cells. In Fig. 2, the peak maxima for the LiBOB and
cells. In Fig. 2, the peak maxima for the LiBOB and  cells are at 1.75 and
cells are at 1.75 and  vs
vs  , respectively. These peaks were observed only in the first graphite-lithiation cycle data [i.e., the peaks are associated with a component (or impurity) in the LiBOB and
, respectively. These peaks were observed only in the first graphite-lithiation cycle data [i.e., the peaks are associated with a component (or impurity) in the LiBOB and  salts that is reduced during the first lithiation cycle].
salts that is reduced during the first lithiation cycle].
Figure 1. First-cycle lithiation–delithiation data for BF graphite∥Li cells containing various electrolytes. The  -axis shows graphite capacity in milliampere hours per gram and the
-axis shows graphite capacity in milliampere hours per gram and the  -axis displays the cell voltage.
-axis displays the cell voltage.
Figure 2. Differential capacity plots, for data shown in Fig. 1, of BF graphite∥Li cells containing various electrolytes. The  -axis voltage range is limited to highlight electrolyte reduction processes. The main graphite lithiation–delithiation peaks, at voltages less than
-axis voltage range is limited to highlight electrolyte reduction processes. The main graphite lithiation–delithiation peaks, at voltages less than  , are beyond the scale of this plot.
, are beyond the scale of this plot.
All cells show voltage plateaus in the capacity plots (Fig. 1) or peaks in the differential capacity plots (Fig. 2) in the  range, corresponding to electrolyte reduction on the graphite negative electrode. These peaks are also observed only during the first graphite-lithiation cycle (i.e., corresponding peaks are not observed during graphite delithiation). In Fig. 2, the peak maximum for the
range, corresponding to electrolyte reduction on the graphite negative electrode. These peaks are also observed only during the first graphite-lithiation cycle (i.e., corresponding peaks are not observed during graphite delithiation). In Fig. 2, the peak maximum for the  cell is at
cell is at  vs
vs  . The
. The  cell displays a wider and deeper peak with a shoulder around
cell displays a wider and deeper peak with a shoulder around  and a maximum at
and a maximum at  . For the LiBOB and
. For the LiBOB and  cells, it is apparent that the high-voltage
cells, it is apparent that the high-voltage  reduction does not suppress further electrolyte reduction on the graphite electrode. The LiBOB electrolyte cells show a small wide peak centered about
reduction does not suppress further electrolyte reduction on the graphite electrode. The LiBOB electrolyte cells show a small wide peak centered about  and a larger, broad peak centered about
and a larger, broad peak centered about  . The
. The  cell also shows a broad peak with a maximum about
cell also shows a broad peak with a maximum about  vs
vs  . These differences in data for the various electrolytes apparently reflect differences in the SEI formation processes.
. These differences in data for the various electrolytes apparently reflect differences in the SEI formation processes.
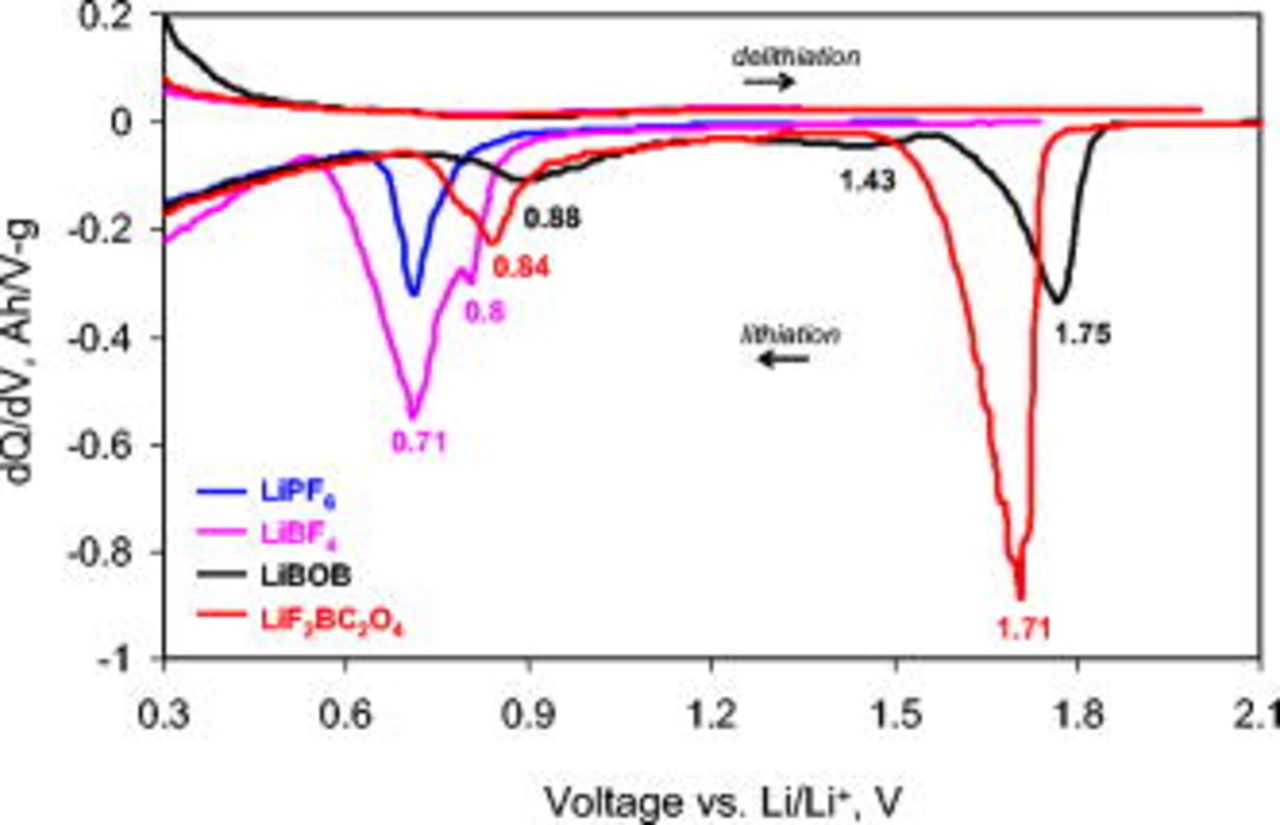
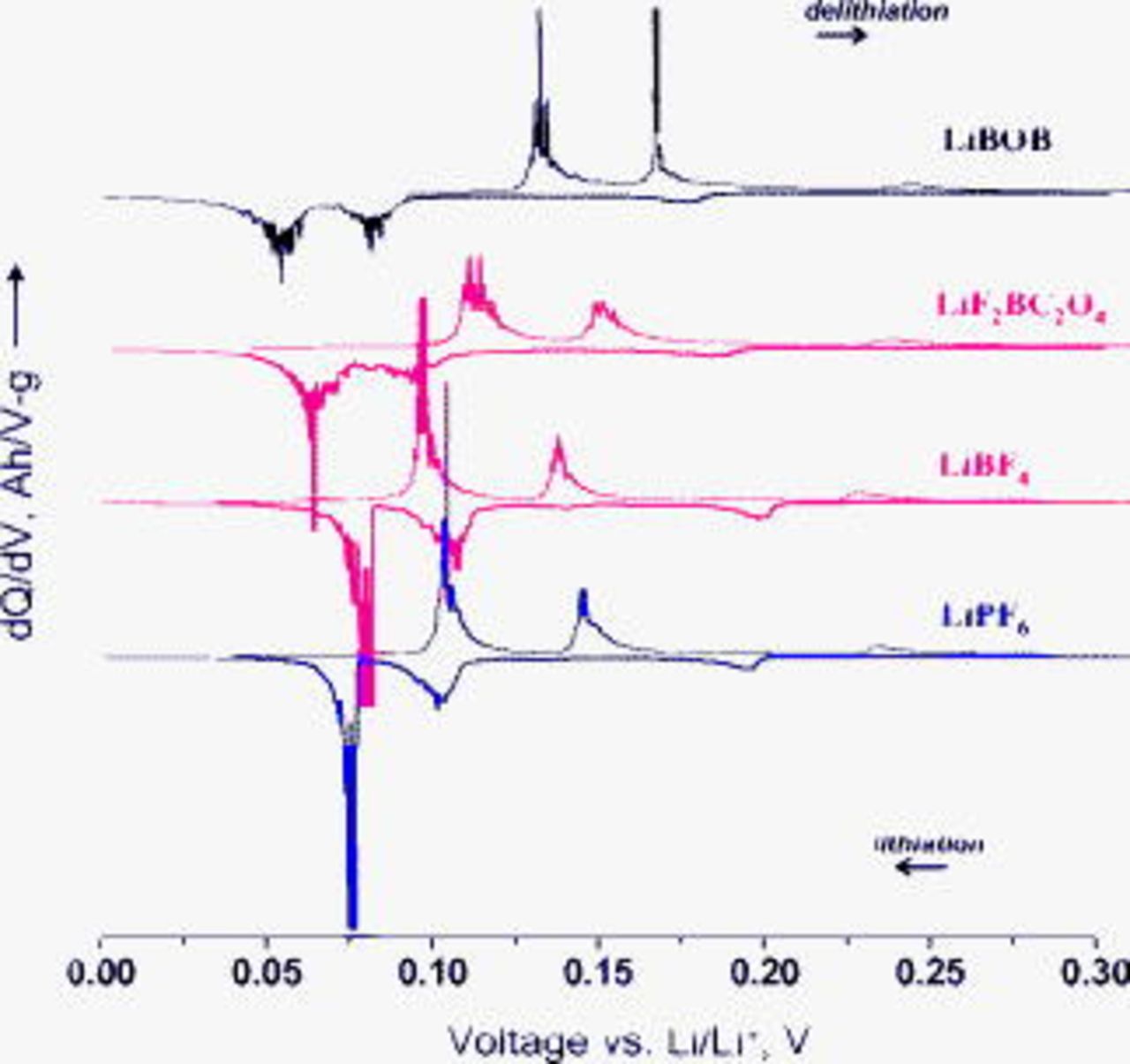
All cells exhibited the expected graphite "staging" behavior below  vs
vs  because of the formation of various
because of the formation of various  phases
phases  by lithium intercalation.31, 32 The graphite staging behavior is seen as peaks in the differential capacity plots (Fig. 3). There are obvious differences between the lithium intercalation–deintercalation behavior in the various electrolytes. For example, the LiBOB cell shows stage 1 and 2 peaks at the lowest voltage during lithium insertion and at the highest voltage during lithium extraction. Figure 3 also shows the asymmetric nature of the lithium insertion∕extraction process; the stage 1 lithium extraction peak is observed at a higher voltage than the stage 1 peak associated with lithium insertion. The differences in the voltage profiles during cycling may arise from several reasons that include (i) differences in electrolyte composition, (ii) sample-to-sample variations that produce differences in electrode impedance, etc.
by lithium intercalation.31, 32 The graphite staging behavior is seen as peaks in the differential capacity plots (Fig. 3). There are obvious differences between the lithium intercalation–deintercalation behavior in the various electrolytes. For example, the LiBOB cell shows stage 1 and 2 peaks at the lowest voltage during lithium insertion and at the highest voltage during lithium extraction. Figure 3 also shows the asymmetric nature of the lithium insertion∕extraction process; the stage 1 lithium extraction peak is observed at a higher voltage than the stage 1 peak associated with lithium insertion. The differences in the voltage profiles during cycling may arise from several reasons that include (i) differences in electrolyte composition, (ii) sample-to-sample variations that produce differences in electrode impedance, etc.
Figure 3. Differential capacity plots showing peaks associated with graphite lithiation–delithiation during the first cycle for BF graphite∥Li cells containing various electrolytes.
In general, the irreversible capacities of the BF graphite electrodes, during the first lithiation–delithiation cycle, are higher than those of traditional composite graphite electrodes. The voltage plateaus during the first lithiation cycle for the BF graphite and composite electrodes appear at the same voltage values for the various electrolytes. However, the plateaus are broader for the BF electrodes probably because of their higher electrochemical surface areas and, therefore, a larger number of graphite sites available for electrolyte decomposition. (The coverage of graphite particles by the PVdF binder in traditional graphite electrodes probably hinders electrolyte decomposition at some of the graphite sites thus reducing the width of the voltage plateau.) The high irreversible capacities of the BF graphite electrodes were observed only during the first cycle. In general, the cycling efficiencies were high (between 95 and 99%) during the subsequent cycles, and the electrodes displayed stable cycling characteristics up to  .15
.15
XPS
XPS data from samples exposed to the various electrolytes are shown in Fig. 4, 5, 6 and 7; the spectral intensities are normalized to highlight peaks in the data. The binding energies (BEs) of the various electrolyte salts are shown in Table I. Variations are often observed between XPS data obtained on the pure salts by different investigators.33, 34 These variations may arise from several reasons that include (i) differential charging, in which BE shifts are induced by the presence of insulating species at the surfaces of conducting electrodes, and (ii) differences in charge-referencing used by the researchers.
Table I. BEs of various electrolyte salts determined by XPS. The data were calibrated based on the C 1s hydrocarbon peak at  .
.
| C 1s | O 1s | F 1s | B 1s | P 
| |
|---|---|---|---|---|---|

| 687.7 | 138.0 | |||

| 687.2 | 195.4 | |||

| 289.8 | 533.7, 532.7 | 193.7 | ||

| 290 | 533.9, 532.9 | 687.2 | 194.7 |
In general, the BF graphite data are simpler to interpret than typical composite electrode data because they do not contain binder peaks in the C 1s and F 1s data. For example, electrodes containing carboxymethyl cellulose display peaks at 286 and  from C–O and
from C–O and  environments in the binder.4 Electrodes containing PVdF display peaks at 286 and
environments in the binder.4 Electrodes containing PVdF display peaks at 286 and  in C 1s and at
in C 1s and at  in F 1s from C–H and C–F environments, respectively, in the binder.35 In addition, the PVdF binder in composite electrodes is known to soak up the carbonate-rich electrolyte; therefore, carbonate peaks could be intense in the XPS data of composite electrodes. Then again, the overlap of the carbonate peak position in the
in F 1s from C–H and C–F environments, respectively, in the binder.35 In addition, the PVdF binder in composite electrodes is known to soak up the carbonate-rich electrolyte; therefore, carbonate peaks could be intense in the XPS data of composite electrodes. Then again, the overlap of the carbonate peak position in the  to 291 eV range with the C–F peak position in the PVdF binder complicates the analysis.
to 291 eV range with the C–F peak position in the PVdF binder complicates the analysis.
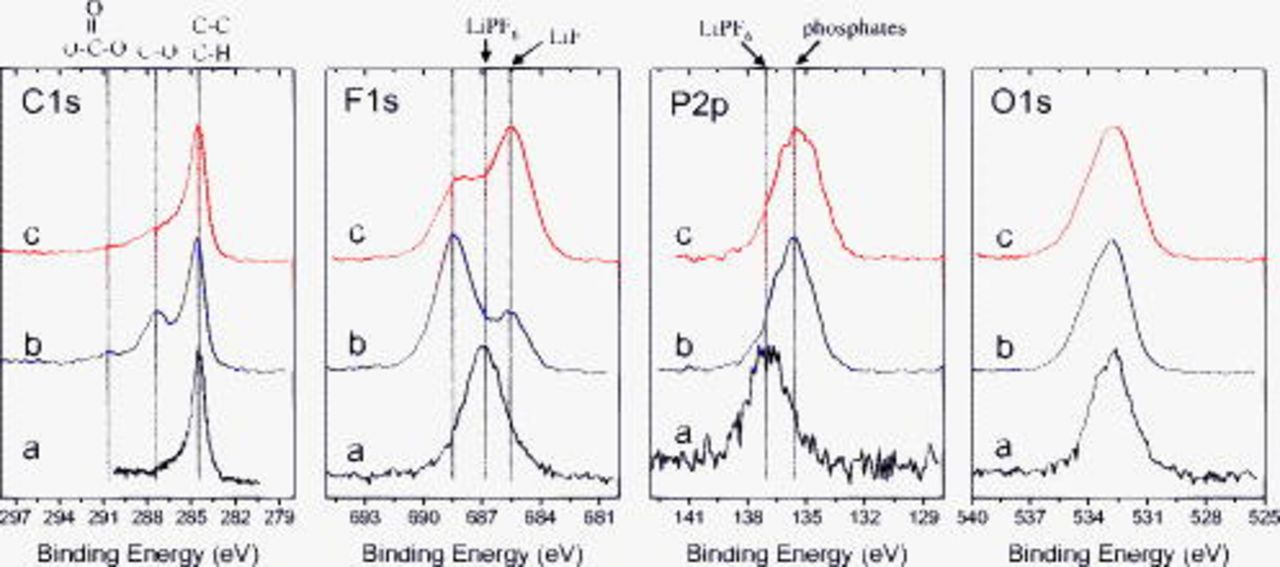
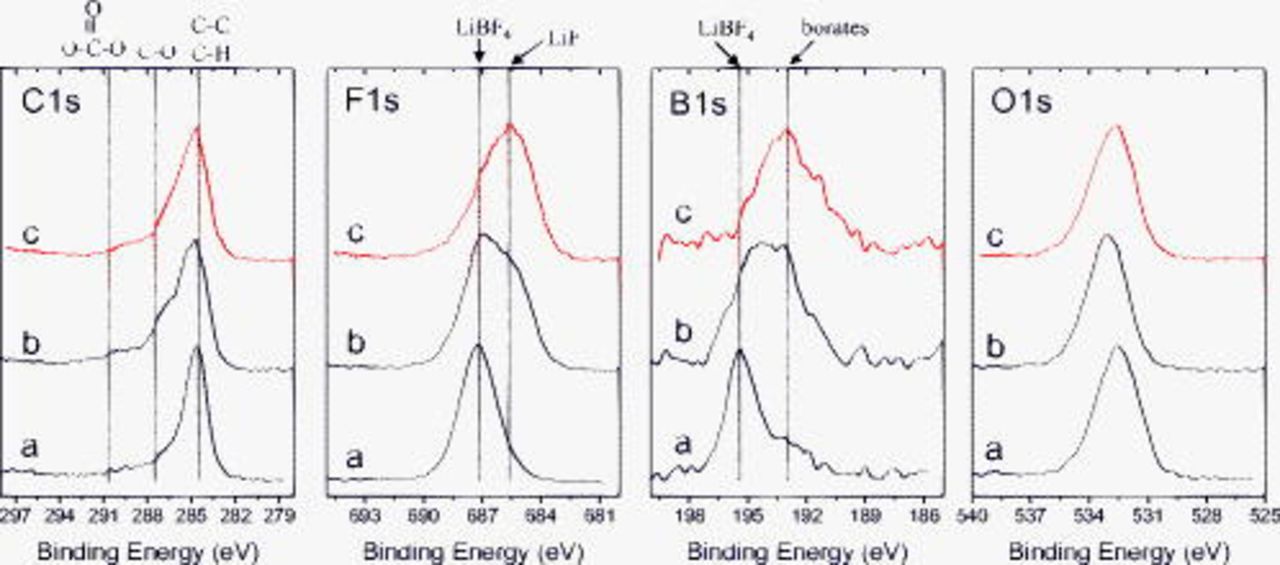
Figure 4 shows C 1s, F 1s, P 2p, and O 1s spectra from the BF graphite electrodes after exposure to  electrolyte. The elemental concentrations calculated from the XPS spectra are given in Table II. The dipped samples show peaks associated with graphite (284.5, C 1s) and intensities associated with the electrolyte components. Interestingly, the F 1s and P 2p BE's are observed at 686.9 and
electrolyte. The elemental concentrations calculated from the XPS spectra are given in Table II. The dipped samples show peaks associated with graphite (284.5, C 1s) and intensities associated with the electrolyte components. Interestingly, the F 1s and P 2p BE's are observed at 686.9 and  , respectively, which are different from the values reported for pure
, respectively, which are different from the values reported for pure  in Table I. These differences may be due to solvation of
in Table I. These differences may be due to solvation of  by solvent (EC) molecules, or may arise from differential charging of
by solvent (EC) molecules, or may arise from differential charging of  . The unrinsed samples indicate peaks associated with alkanes (C–H,
. The unrinsed samples indicate peaks associated with alkanes (C–H,  ), ethers or lithium alkoxides (C–O,
), ethers or lithium alkoxides (C–O,  ), and carbonates
), and carbonates  in the C 1s spectrum, in addition to the graphite peak; these additional compounds are electrolyte reduction products generated during the electrochemical cycling. The F 1s spectrum indicates peaks associated with P–F and O–P–F
in the C 1s spectrum, in addition to the graphite peak; these additional compounds are electrolyte reduction products generated during the electrochemical cycling. The F 1s spectrum indicates peaks associated with P–F and O–P–F  , presumably from fluorophosphate and oxyfluorophosphate compounds, and LiF
, presumably from fluorophosphate and oxyfluorophosphate compounds, and LiF  formed during cycling. The
formed during cycling. The  and
and  species in the unrinsed sample are obviously different from the
species in the unrinsed sample are obviously different from the  electrolyte residue in the dipped sample, as indicated by the difference in F 1s BEs. The peak at
electrolyte residue in the dipped sample, as indicated by the difference in F 1s BEs. The peak at  in the P 2p spectrum most likely arises from P–O functional groups in the oxyfluorophosphate compounds.36 Rinsing in EMC exposes more of the graphite by dissolving electrolyte residue and portions of the SEI. Rinsing decreases the alkoxide and carbonate content in the C 1s spectra, decreases P–F and O–P–F content but increases LiF content in the F 1s spectra, and decreases the P–O content in the P 2p spectra. The reduction in relative oxygen concentration (see Table II) on rinsing is consistent with the removal of oxygen-bearing species.
in the P 2p spectrum most likely arises from P–O functional groups in the oxyfluorophosphate compounds.36 Rinsing in EMC exposes more of the graphite by dissolving electrolyte residue and portions of the SEI. Rinsing decreases the alkoxide and carbonate content in the C 1s spectra, decreases P–F and O–P–F content but increases LiF content in the F 1s spectra, and decreases the P–O content in the P 2p spectra. The reduction in relative oxygen concentration (see Table II) on rinsing is consistent with the removal of oxygen-bearing species.
Figure 4. XPS data from BF graphite samples exposed to  electrolyte: (a) dipped, then dried, (b) cycled, but unrinsed, and (c) EMC rinsed.
electrolyte: (a) dipped, then dried, (b) cycled, but unrinsed, and (c) EMC rinsed.
Table II. Element concentrations (in atomic percent) calculated from XPS spectra of dipped, unrinsed, and rinsed BF graphite samples exposed to various electrolytes.
| Salt | Electrodes | Li 1s | B 1s | C 1s | O 1s | F 1s | P 2p |
|---|---|---|---|---|---|---|---|

| Dipped | 6.8 | – | 19 | 32 | 37.3 | 4.9 |
| Unrinsed | 24.1 | – | 23 | 27.2 | 19.4 | 6.3 | |
| Rinsed | 26.1 | – | 39.2 | 14.4 | 17 | 3.3 | |

| Dipped | 13.4 | 10.8 | 23.1 | 12.6 | 40.1 | – |
| Unrinsed | 15.4 | 9.3 | 29.9 | 18.3 | 27.1 | – | |
| Rinsed | 11.2 | 8.1 | 45 | 22.4 | 13.3 | – | |
| Dipped | 3.2 | 2.8 | 57.7 | 28.2 | 8.1 | – |
| Unrinsed | 14.7 | 6.7 | 36.6 | 31.6 | 10.4 | – | |
| Rinsed | 26.6 | 4.4 | 31.3 | 23.1 | 14.6 | – | |
| LiBOB | Dipped | 9.2 | 0.8 | 68.9 | 21.1 | – | – |
| Unrinsed | 8.3 | 5.5 | 42 | 44.2 | – | – | |
| Rinsed | 8.5 | 0.3 | 49.6 | 41.6 | – | – |
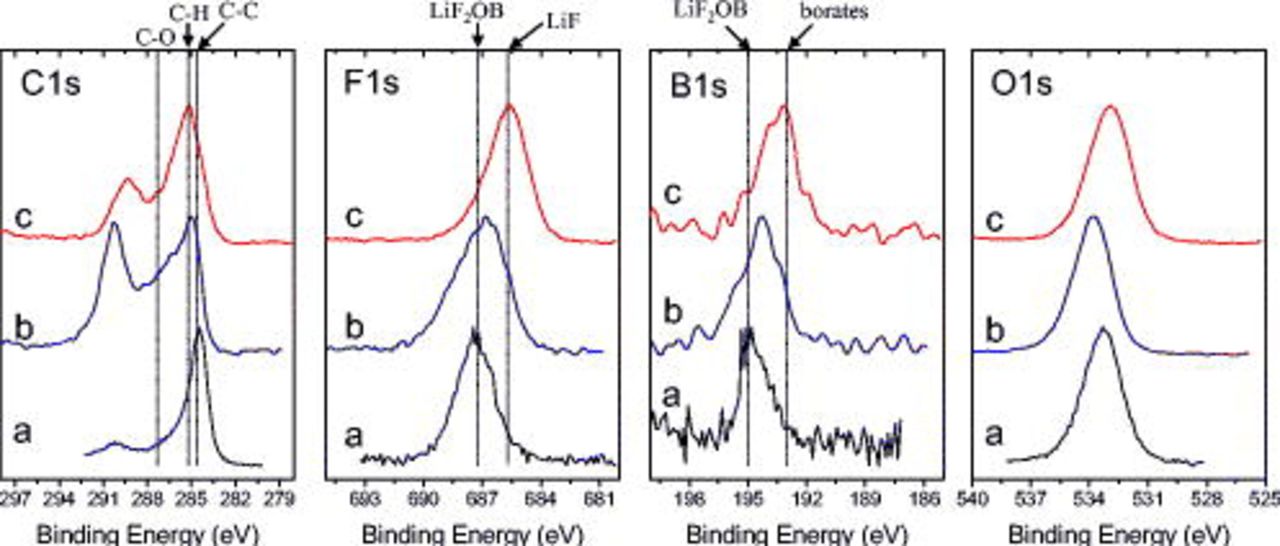
Figure 5 shows C 1s, F 1s, B 1s, and O 1s spectra from the BF graphite electrodes after exposure to the  electrolyte. The dipped samples showed high Li, B, and F concentrations, indicating
electrolyte. The dipped samples showed high Li, B, and F concentrations, indicating  residue (
residue ( , F 1s;
, F 1s;  , B 1s) on the graphite surface. The cycled but unrinsed samples indicate intensities for C–H
, B 1s) on the graphite surface. The cycled but unrinsed samples indicate intensities for C–H  , ethers or lithium alkoxides
, ethers or lithium alkoxides  , and carbonates
, and carbonates  compounds in the C 1s spectrum, in addition to graphite. The F 1s spectrum indicates intensities associated with B–F and O–B–F
compounds in the C 1s spectrum, in addition to graphite. The F 1s spectrum indicates intensities associated with B–F and O–B–F  , and LiF
, and LiF  formed during cycling. The intensities at
formed during cycling. The intensities at  in the B 1s spectrum arises from the B–F and O–B–F compounds. Rinsing in EMC exposes more of the graphite by washing off some of the surface compounds, including solvated
in the B 1s spectrum arises from the B–F and O–B–F compounds. Rinsing in EMC exposes more of the graphite by washing off some of the surface compounds, including solvated  species. Table II shows significant decreases in the relative concentrations of Li and F, and an increase in the oxygen concentration. The intensity of the
species. Table II shows significant decreases in the relative concentrations of Li and F, and an increase in the oxygen concentration. The intensity of the  peak in the C 1s spectrum is not altered much by EMC washing, which suggests that the ether linkages probably result from inorganic C–O–B compounds. The rinsed sample intensities are consistent with the presence of LiF,
peak in the C 1s spectrum is not altered much by EMC washing, which suggests that the ether linkages probably result from inorganic C–O–B compounds. The rinsed sample intensities are consistent with the presence of LiF,  , and
, and  species on the sample surface.
species on the sample surface.
Figure 5. XPS data from BF graphite samples exposed to  electrolyte: (a) dipped, then dried, (b) cycled, but unrinsed, and (c) EMC rinsed.
electrolyte: (a) dipped, then dried, (b) cycled, but unrinsed, and (c) EMC rinsed.
Figure 6 shows C 1s, F 1s, B 1s, and O 1s spectra from the BF graphite electrodes after exposure to the  electrolyte. The dipped sample data contain peaks associated with the
electrolyte. The dipped sample data contain peaks associated with the  salt (
salt ( , C 1s;
, C 1s;  , F 1s;
, F 1s;  , B 1s) along with graphite. The C 1s spectrum of the cycled but unrinsed sample shows significant intensities associated with C–H
, B 1s) along with graphite. The C 1s spectrum of the cycled but unrinsed sample shows significant intensities associated with C–H  , C–O (ethers or lithium alkoxides, 287.2), and oxalate
, C–O (ethers or lithium alkoxides, 287.2), and oxalate  species; the intensity observed in the
species; the intensity observed in the  range is from carbonates. The absence of the
range is from carbonates. The absence of the  peak indicates that graphite is buried under overlying compounds in the SEI. Significant changes are observed in the data after EMC rinsing. The increase in the relative concentrations of Li and F, and the intensity at
peak indicates that graphite is buried under overlying compounds in the SEI. Significant changes are observed in the data after EMC rinsing. The increase in the relative concentrations of Li and F, and the intensity at  in F 1s, indicates that more LiF is exposed after rinsing. The C 1s spectrum after rinsing showed an increase in the relative intensity at
in F 1s, indicates that more LiF is exposed after rinsing. The C 1s spectrum after rinsing showed an increase in the relative intensity at  (C–H), but a decrease in the relative intensity at
(C–H), but a decrease in the relative intensity at  (oxalates). However, the data still shows a significant peak at
(oxalates). However, the data still shows a significant peak at  , which is associated with lithium oxalate (or alkyl esters of oxalic acid). The graphite peak is not observed even after rinsing, which suggests that the SEI contains carbon-bearing (oligomeric) species that are not soluble in EMC. The B 1s spectra are consistent with the presence of
, which is associated with lithium oxalate (or alkyl esters of oxalic acid). The graphite peak is not observed even after rinsing, which suggests that the SEI contains carbon-bearing (oligomeric) species that are not soluble in EMC. The B 1s spectra are consistent with the presence of  and
and  compounds in the SEI.
compounds in the SEI.
Figure 6. XPS data from BF graphite samples exposed to  electrolyte: (a) dipped, then dried, (b) cycled, but unrinsed, and (c) EMC rinsed.
electrolyte: (a) dipped, then dried, (b) cycled, but unrinsed, and (c) EMC rinsed.
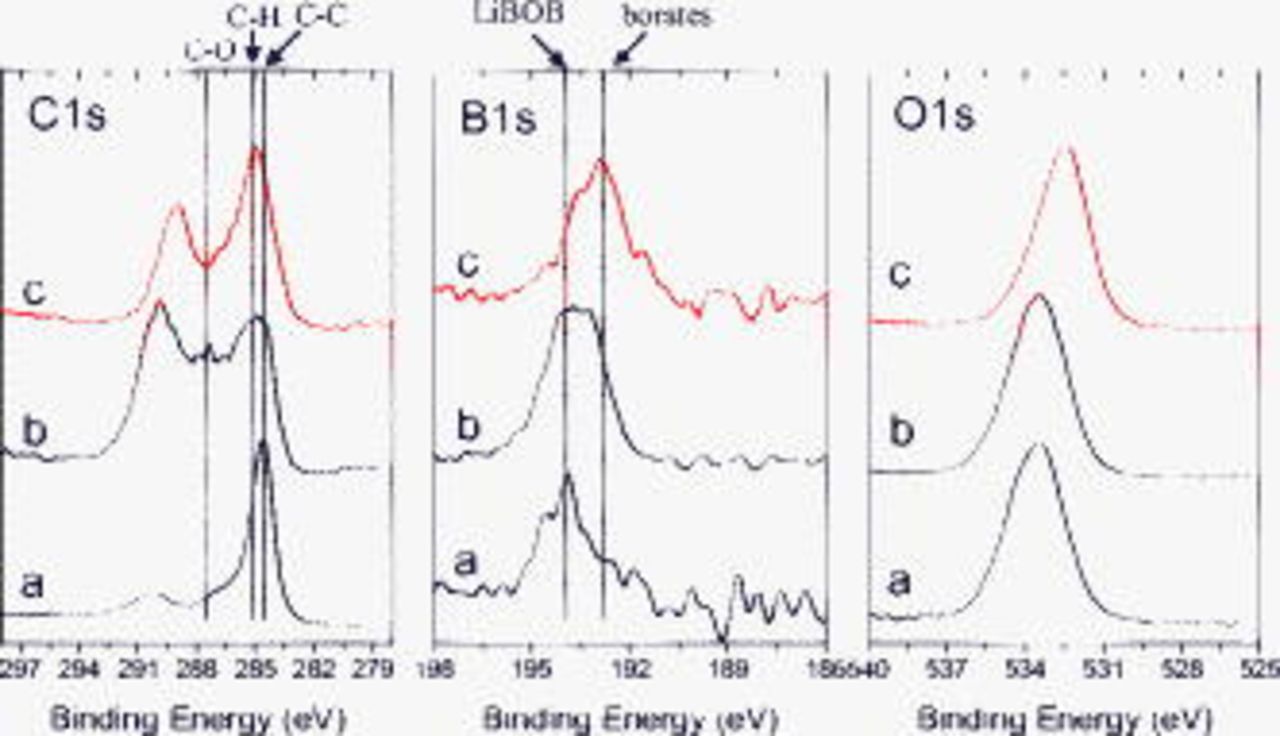
Figure 7 shows C 1s, B 1s, and O 1s spectra from the BF graphite electrodes after exposure to the LiBOB electrolyte. The dipped sample data contain peaks associated with the LiBOB salt ( , C 1s;
, C 1s;  , B 1s), and graphite. The
, B 1s), and graphite. The  peak is not observed in data from the unrinsed and rinsed samples, which indicates that the graphite is buried under overlying compounds in the SEI. The C 1s spectrum of the cycled but unrinsed sample shows significant intensities associated with C–H
peak is not observed in data from the unrinsed and rinsed samples, which indicates that the graphite is buried under overlying compounds in the SEI. The C 1s spectrum of the cycled but unrinsed sample shows significant intensities associated with C–H  , C–O (ethers or lithium alkoxides,
, C–O (ethers or lithium alkoxides,  ), and oxalate
), and oxalate  species. On EMC rinsing, the relative concentration of C increases, whereas the relative concentrations of B and O decrease (see Table II). The C 1s spectrum after rinsing shows an increase in the relative intensity at
species. On EMC rinsing, the relative concentration of C increases, whereas the relative concentrations of B and O decrease (see Table II). The C 1s spectrum after rinsing shows an increase in the relative intensity at  (C–H), but a decrease in the relative intensities at 287.5 and
(C–H), but a decrease in the relative intensities at 287.5 and  . Moreover, the data shows a significant peak at
. Moreover, the data shows a significant peak at  , which is associated with lithium oxalate (or alkyl esters of oxalic acid).
, which is associated with lithium oxalate (or alkyl esters of oxalic acid).
Figure 7. XPS data from BF graphite samples exposed to LiBOB electrolyte: (a) dipped, then dried, (b) cycled, but unrinsed, and (c) EMC rinsed.
FTIR
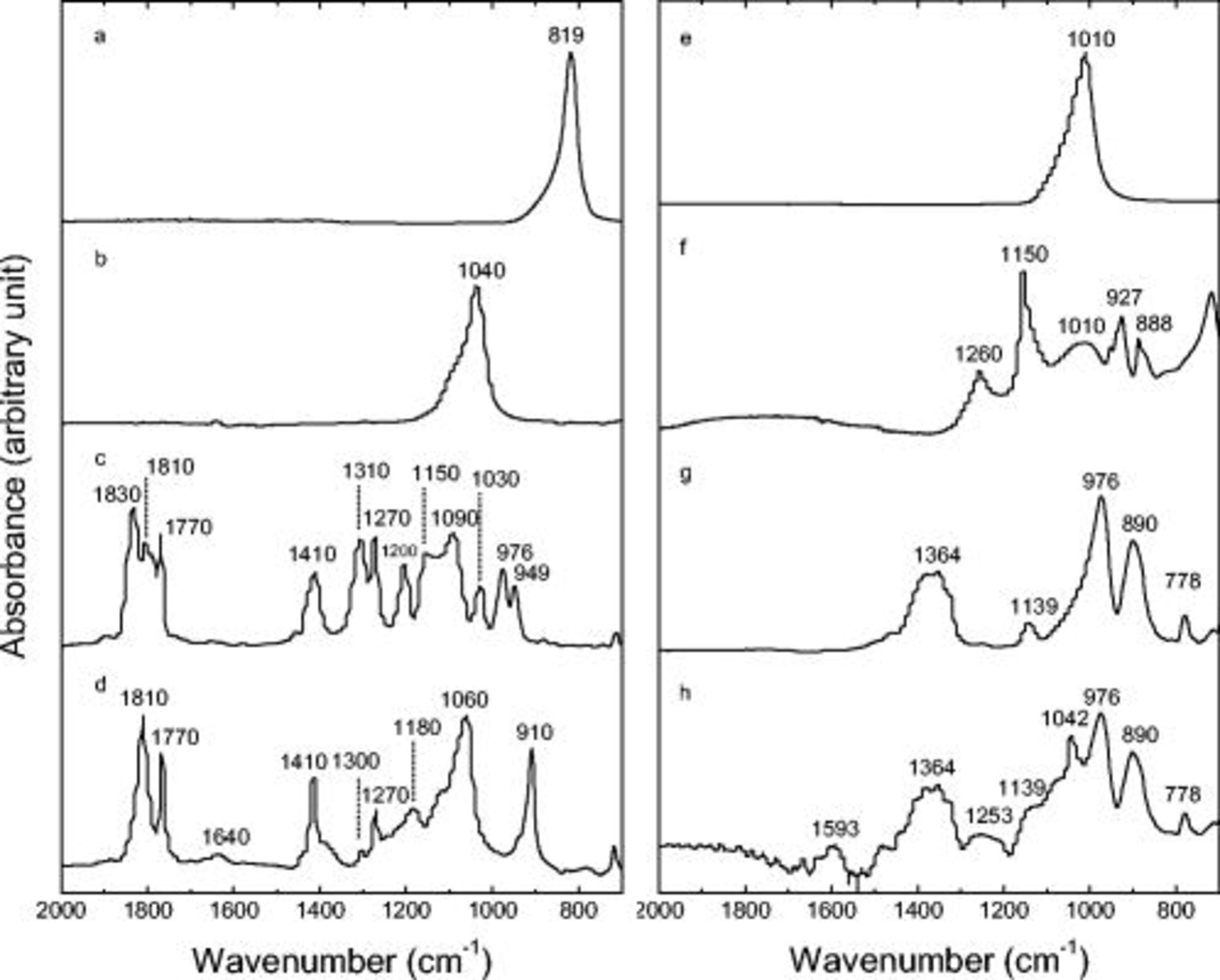
FTIR data from the samples exposed to the various electrolytes are shown in Fig. 8, 9, 10 and 11. The spectra contain numerous peaks that reflect the presence of organic species on the graphite. A rigorous analysis of these spectra is not possible because several possible species in the SEI have overlapping IR bands. We have attempted to identify possible compounds in the SEI by comparing the BF graphite electrode results with data available in literature8, 37 and with model compound data that we gathered during the course of this study (see Fig. 12). The  ,
,  ,
,  , LiBOB,
, LiBOB,  , and
, and  compounds were acquired from commercial suppliers. The
compounds were acquired from commercial suppliers. The  compound was prepared by a solid-state reaction of
compound was prepared by a solid-state reaction of  and
and  , and the
, and the  compound was prepared by reacting
compound was prepared by reacting  and
and  .
.
Figure 12. FTIR data from (a)  , (b)
, (b)  , (c) LiBOB, (d)
, (c) LiBOB, (d)  , (e)
, (e)  , (f)
, (f)  , (g)
, (g)  , and (h)
, and (h)  .
.
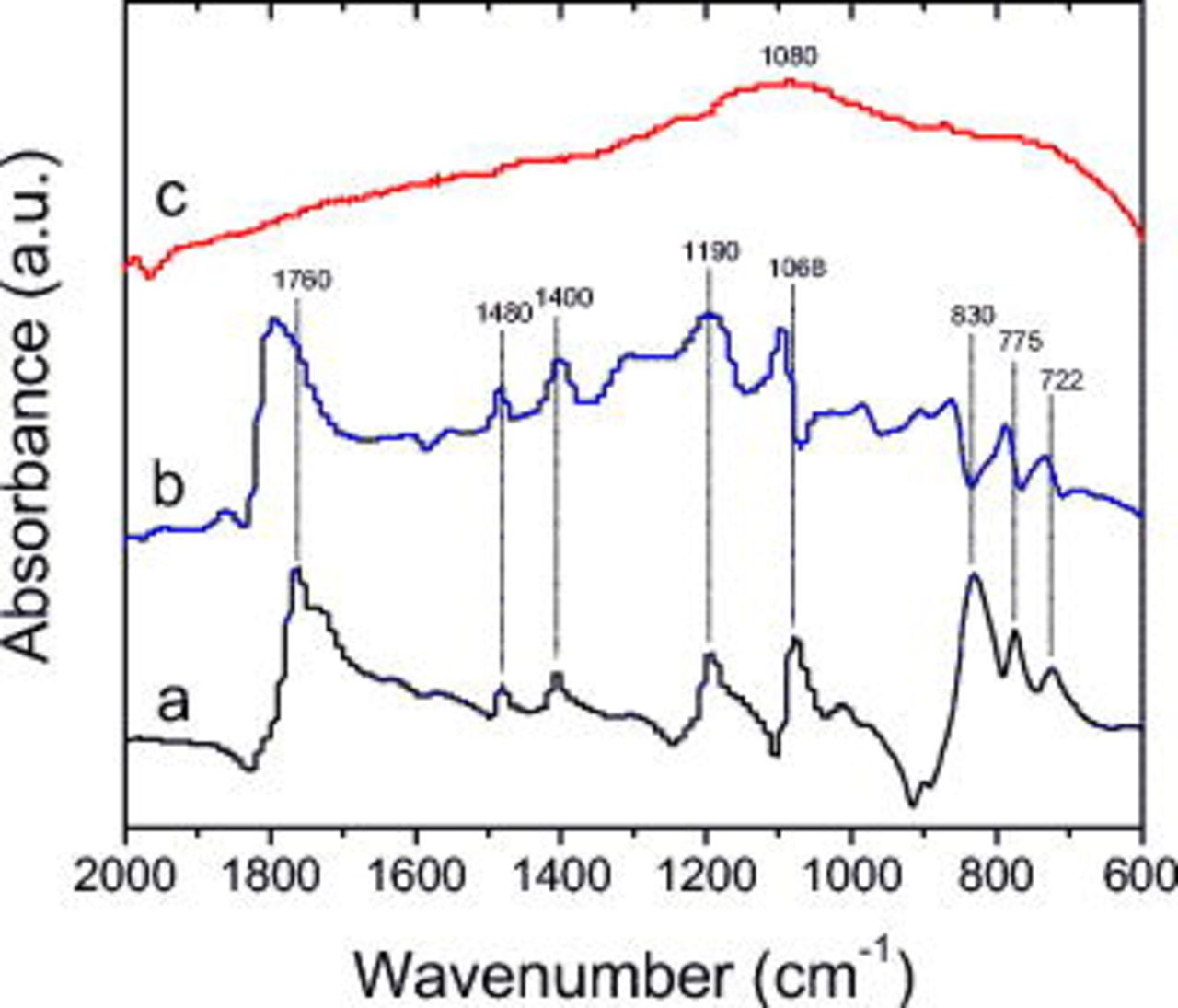
Figure 8 contains spectra from electrodes exposed to the  electrolyte. The dipped sample contains absorption peaks at 1760
electrolyte. The dipped sample contains absorption peaks at 1760  , 1560, 1480
, 1560, 1480  , 1400
, 1400  , 1190, 1068
, 1190, 1068  , 830
, 830  , 771
, 771  , and
, and  . By comparison to "model" data from EC and
. By comparison to "model" data from EC and  , these peaks can be attributed to
, these peaks can be attributed to  solvates. The unrinsed sample data are similar to the dipped sample data, except that most absorptions appear to be shifted to higher wavenumbers. IR absorptions are fairly sensitive to environment. For example, if a
solvates. The unrinsed sample data are similar to the dipped sample data, except that most absorptions appear to be shifted to higher wavenumbers. IR absorptions are fairly sensitive to environment. For example, if a  bond is coordinated to a metal such as Li, then this will shift the absorption due to a change in the bond-stretching frequency. The small shifts that are observed between the dipped and unrinsed spectra may be due to small differences in the environment of the samples (more or less Li ions, slight difference in charge of graphite, etc.). The presence of lithium alkyl carbonates cannot be confirmed in the data; absorptions from compounds such as lithium ethylene dicarbonate, if present, may be overshadowed by strong absorptions from the
bond is coordinated to a metal such as Li, then this will shift the absorption due to a change in the bond-stretching frequency. The small shifts that are observed between the dipped and unrinsed spectra may be due to small differences in the environment of the samples (more or less Li ions, slight difference in charge of graphite, etc.). The presence of lithium alkyl carbonates cannot be confirmed in the data; absorptions from compounds such as lithium ethylene dicarbonate, if present, may be overshadowed by strong absorptions from the  solvates. The peak at
solvates. The peak at  is not attributed to
is not attributed to  because
because  also shows a very strong absorption at
also shows a very strong absorption at  , which is not observed.38, 39 This observation is in accordance with previous studies, which concluded that
, which is not observed.38, 39 This observation is in accordance with previous studies, which concluded that  in the graphite SEI can be avoided by appropriate cell operation and handling of the electrodes.6, 15 The absorptions in the
in the graphite SEI can be avoided by appropriate cell operation and handling of the electrodes.6, 15 The absorptions in the  region may be from compounds containing C–O and P–O functional groups, such as lithium alkoxides, lithium alkyl phosphates, or lithium fluorophosphates. In the rinsed sample, the single broad adsorption at
region may be from compounds containing C–O and P–O functional groups, such as lithium alkoxides, lithium alkyl phosphates, or lithium fluorophosphates. In the rinsed sample, the single broad adsorption at  is attributed to lithium alkyl phosphates
is attributed to lithium alkyl phosphates  (
( group) or lithium fluorophosphates
group) or lithium fluorophosphates  .
.
Figure 8. FTIR data from BF graphite samples exposed to  electrolyte: (a) dipped, then dried, (b) cycled, but unrinsed, and (c) EMC rinsed.
electrolyte: (a) dipped, then dried, (b) cycled, but unrinsed, and (c) EMC rinsed.
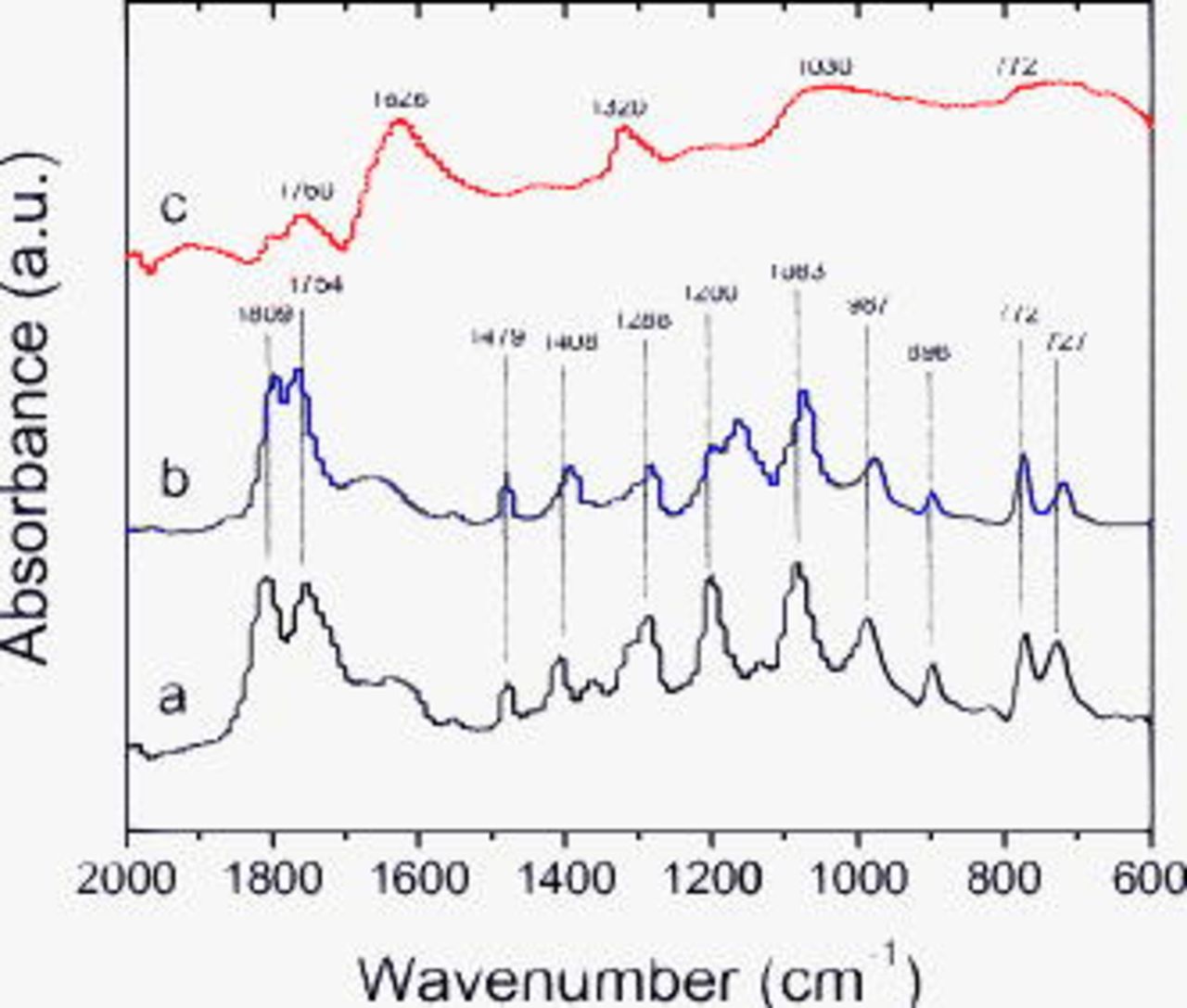
Figure 9 contains spectra from electrodes exposed to the  electrolyte. The main absorptions of the
electrolyte. The main absorptions of the  solvates on the dipped electrode are at 1762
solvates on the dipped electrode are at 1762  , 1482
, 1482  , 1407
, 1407  , 1191
, 1191  , 1052
, 1052  , 977
, 977  , 901
, 901  , and 771
, and 771  and
and  . Although there are small differences in intensities, the absorptions of the unrinsed and dipped electrode are relatively similar. Furthermore, the additional absorptions in the unrinsed sample are similar to the absorptions that are retained after rinsing the electrode, which indicates that sample rinsing removes most of the residual
. Although there are small differences in intensities, the absorptions of the unrinsed and dipped electrode are relatively similar. Furthermore, the additional absorptions in the unrinsed sample are similar to the absorptions that are retained after rinsing the electrode, which indicates that sample rinsing removes most of the residual  solvate. The rinsed electrode spectrum supports the existence of B–O or B–F linkages in borates or fluoroborates (broad peak
solvate. The rinsed electrode spectrum supports the existence of B–O or B–F linkages in borates or fluoroborates (broad peak  ) and alkyl orthoborates such as
) and alkyl orthoborates such as  or
or  (1355, 1260, and
(1355, 1260, and  ).8 The broad peak centered around
).8 The broad peak centered around  suggests the presence of compounds bearing carbonyl bonds. The unrinsed sample has an additional peak at 1160, probably a C–O stretch from either an ether or an alkoxide that is washed away on rinsing. In general, the data show that absorptions characteristic of C–H or
suggests the presence of compounds bearing carbonyl bonds. The unrinsed sample has an additional peak at 1160, probably a C–O stretch from either an ether or an alkoxide that is washed away on rinsing. In general, the data show that absorptions characteristic of C–H or  bonds (other than those from the electrolyte residue) are weak. That is, the graphite SEI generated during cycling in the
bonds (other than those from the electrolyte residue) are weak. That is, the graphite SEI generated during cycling in the  electrolyte shows high concentrations of inorganic borates
electrolyte shows high concentrations of inorganic borates  or lithium fluoroborates
or lithium fluoroborates  and low concentrations of organic compounds on the SEI surface.
and low concentrations of organic compounds on the SEI surface.
Figure 9. FTIR data from BF graphite samples exposed to  electrolyte: (a) dipped, then dried, (b) cycled, but unrinsed, and (c) EMC rinsed.
electrolyte: (a) dipped, then dried, (b) cycled, but unrinsed, and (c) EMC rinsed.
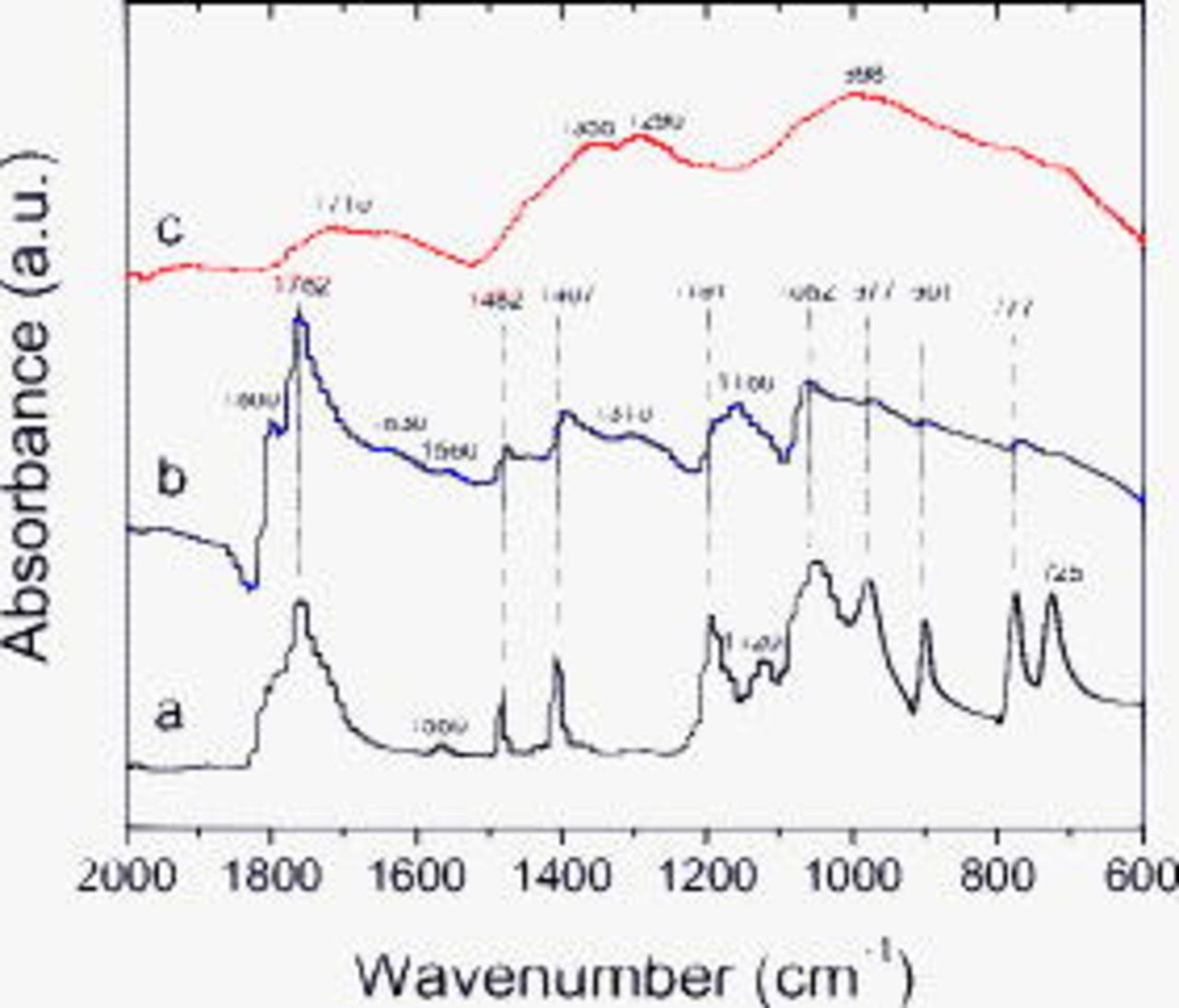
Figure 10 contains spectra from electrodes exposed to the  electrolyte. The main absorptions from
electrolyte. The main absorptions from  solvates are at 1810, 1780
solvates are at 1810, 1780  , 1722
, 1722  , 1480
, 1480  , 1430, 1358
, 1430, 1358  , 1240, 1213
, 1240, 1213  , 1140, 1091
, 1140, 1091  , 1052
, 1052  , 957
, 957  , 904, 784
, 904, 784  , and
, and  . Many of the absorptions in the unrinsed electrode spectrum may be associated with
. Many of the absorptions in the unrinsed electrode spectrum may be associated with  solvates; the remaining resonances are similar to the absorptions observed in the rinsed samples. After EMC rinsing, the remaining surface species on the electrode have absorptions characteristic of lithium oxalate (1633, 1327, and
solvates; the remaining resonances are similar to the absorptions observed in the rinsed samples. After EMC rinsing, the remaining surface species on the electrode have absorptions characteristic of lithium oxalate (1633, 1327, and  ), B–O–B, C–O–B, and B–F linkages (1006, 938, and
), B–O–B, C–O–B, and B–F linkages (1006, 938, and  , respectively), and alkanes. The broad peak centered around
, respectively), and alkanes. The broad peak centered around  suggests the presence of compounds bearing carbonyl bonds. The unrinsed sample has an additional peak at 1160, probably a C–O stretch from either an ether or an alkoxide that is washed away on rinsing. The IR data, in conjunction with the XPS data, support the presence of lithium oxalate (or alkyl esters of oxalic acid), and various B-containing species, including tri-coordinated borates and
suggests the presence of compounds bearing carbonyl bonds. The unrinsed sample has an additional peak at 1160, probably a C–O stretch from either an ether or an alkoxide that is washed away on rinsing. The IR data, in conjunction with the XPS data, support the presence of lithium oxalate (or alkyl esters of oxalic acid), and various B-containing species, including tri-coordinated borates and  in the SEI.
in the SEI.
Figure 10. FTIR data from BF graphite samples exposed to  electrolyte: (a) dipped, then dried, (b) cycled, but unrinsed, and (c) EMC rinsed.
electrolyte: (a) dipped, then dried, (b) cycled, but unrinsed, and (c) EMC rinsed.
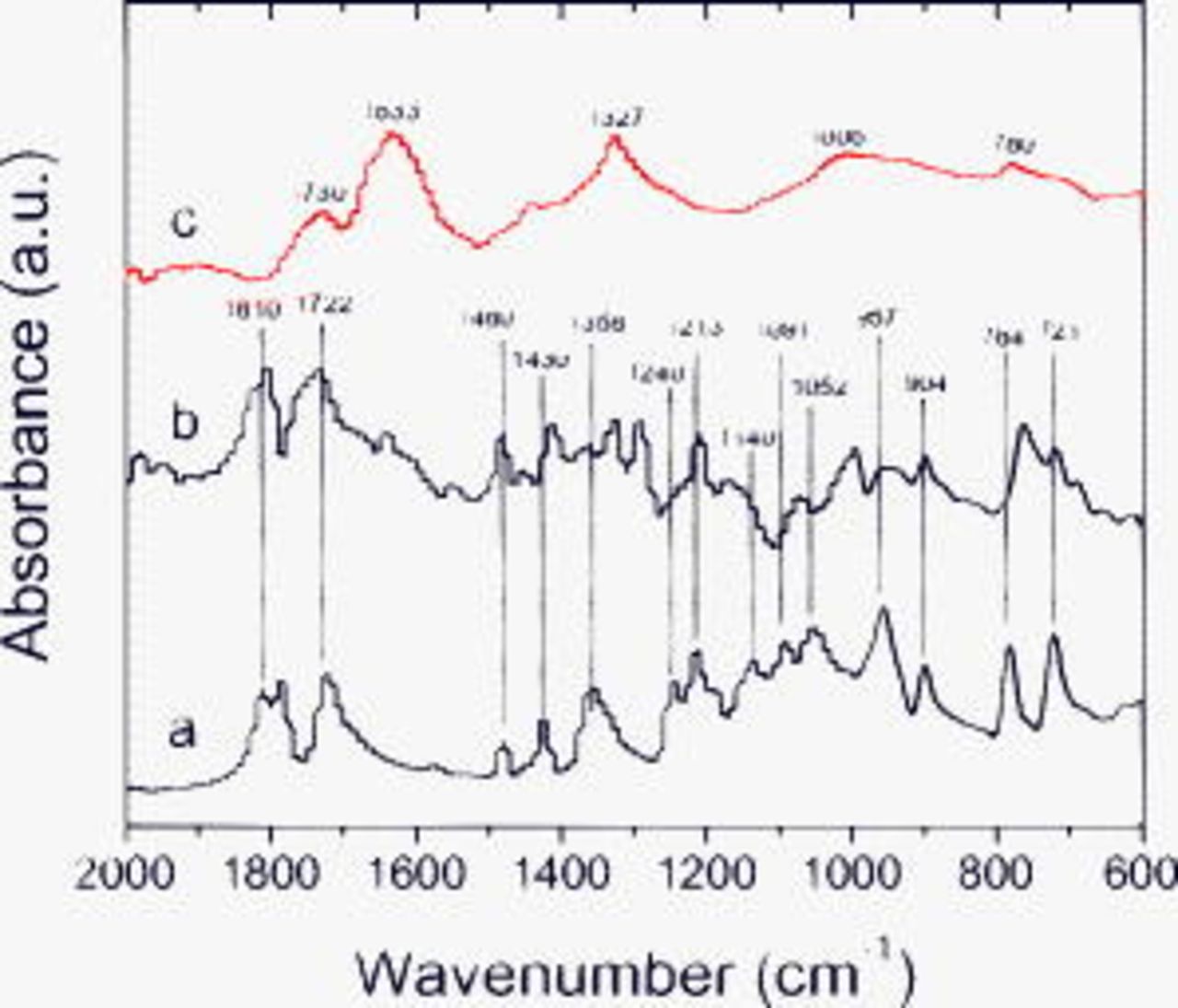
Figure 11 contains spectra from electrodes exposed to the LiBOB electrolyte. The main absorptions from  solvates are at 1809
solvates are at 1809  , 1754
, 1754  , 1479
, 1479  , 1408
, 1408  , 1288
, 1288  , 1200
, 1200  , 1083
, 1083  , 987
, 987  , 898
, 898  , 772
, 772  , and
, and  . The spectrum of the unrinsed electrode is similar to that of the dipped electrode; the additional peak at
. The spectrum of the unrinsed electrode is similar to that of the dipped electrode; the additional peak at  , similar to that observed for
, similar to that observed for  and
and  , is probably a C–O stretch from either an ether or an alkoxide that appears to be washed away on rinsing. Compared to the unrinsed spectrum, the rinsed data contains fewer peaks, which indicates that the electrolyte residue and possibly a portion of the SEI are washed away. The peaks at 1810 and
, is probably a C–O stretch from either an ether or an alkoxide that appears to be washed away on rinsing. Compared to the unrinsed spectrum, the rinsed data contains fewer peaks, which indicates that the electrolyte residue and possibly a portion of the SEI are washed away. The peaks at 1810 and  suggest the presence of compounds bearing carbonyl bonds. Absorptions characteristic of lithium oxalate (1626, 1320, and
suggest the presence of compounds bearing carbonyl bonds. Absorptions characteristic of lithium oxalate (1626, 1320, and  ), B–O–B linkages
), B–O–B linkages  , and hydrocarbons (2913 and
, and hydrocarbons (2913 and  ) are also observed in the data. The rinsed data are similar to that obtained for the
) are also observed in the data. The rinsed data are similar to that obtained for the  sample and support the presence of lithium oxalate (or alkyl esters of oxalic acid), various B-containing species, including tri-coordinated borates and
sample and support the presence of lithium oxalate (or alkyl esters of oxalic acid), various B-containing species, including tri-coordinated borates and  in the SEI.
in the SEI.
Figure 11. FTIR data from BF graphite samples exposed to LiBOB electrolyte: (a) dipped, then dried, (b) cycled, but unrinsed, and (c) EMC rinsed.
TGA
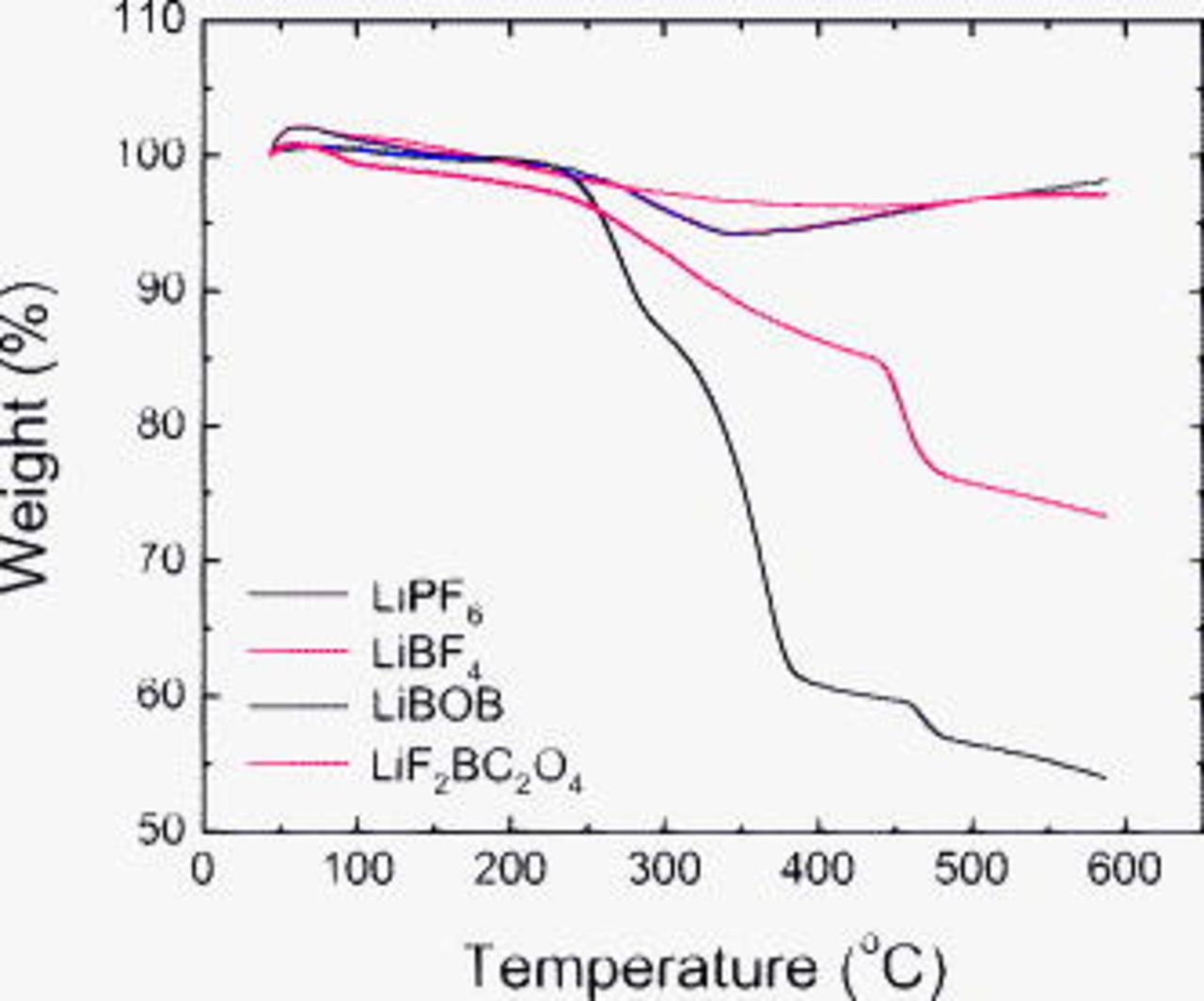
TGA data, obtained on graphite powders from the rinsed electrodes, are shown in Fig. 13 and 14. The  , LiBOB and
, LiBOB and  samples show a small weight gain (up to 2%) on initial heating with a peak maximum around
samples show a small weight gain (up to 2%) on initial heating with a peak maximum around  (Fig. 13), which may be related to reaction of the SEI with oxygen in air. On heating past
(Fig. 13), which may be related to reaction of the SEI with oxygen in air. On heating past  , all samples show gradual and similar weight loss (to
, all samples show gradual and similar weight loss (to  ). The weight-loss characteristics of the various samples differ significantly beyond
). The weight-loss characteristics of the various samples differ significantly beyond  . The weight loss is greatest for the LiBOB sample, which suggests large concentrations of oligomers∕organic species on the electrode surface. In contrast, the weight loss is relatively small for the
. The weight loss is greatest for the LiBOB sample, which suggests large concentrations of oligomers∕organic species on the electrode surface. In contrast, the weight loss is relatively small for the  and
and  samples, which indicates low concentrations of organic compounds in these samples. The
samples, which indicates low concentrations of organic compounds in these samples. The  data show an intermediate weight loss, which suggests that the SEI has less oligomers∕organics than the LiBOB sample but much more than that on the
data show an intermediate weight loss, which suggests that the SEI has less oligomers∕organics than the LiBOB sample but much more than that on the  and
and  samples.
samples.
Figure 13. TGA weight change data from graphite samples removed from EMC-rinsed electrodes that were cycled in various electrolytes.
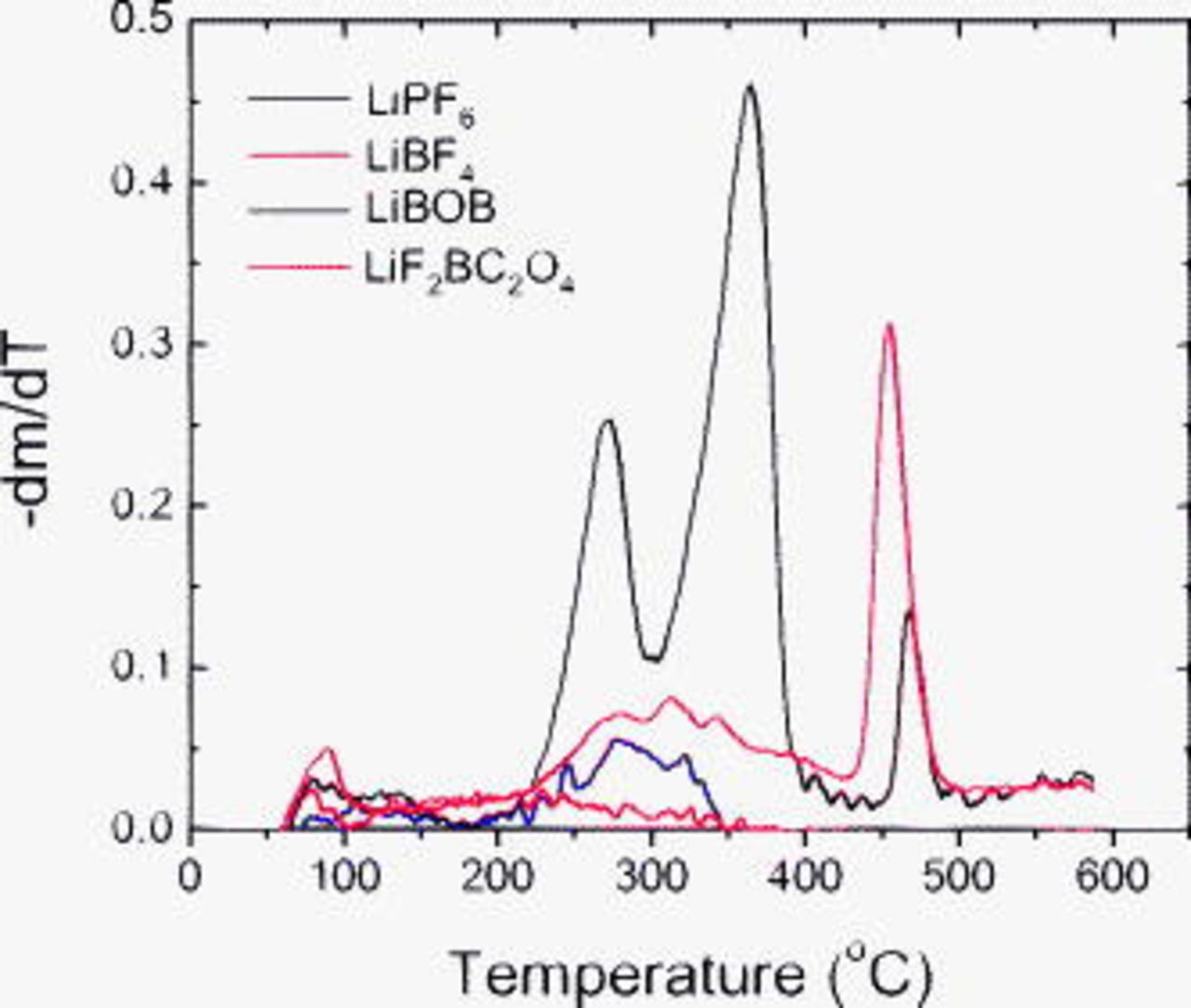
Figure 14, which is a plot of the weight-loss derivative as a function of temperature, provides another perspective on the TGA data. The peaks in the data are associated with the decomposition of SEI component(s) in a particular temperature range. For example, both the LiBOB and  data show a peak
data show a peak  , which can be attributed to the thermal decomposition of lithium oxalate in the SEI. (TGA data from high-purity lithium oxalate was obtained to confirm this assignment.) The LiBOB data also shows distinct peaks centered near
, which can be attributed to the thermal decomposition of lithium oxalate in the SEI. (TGA data from high-purity lithium oxalate was obtained to confirm this assignment.) The LiBOB data also shows distinct peaks centered near  and
and  . The LiBOB salt is known to be thermally stable up to
. The LiBOB salt is known to be thermally stable up to  and loses weight beyond this temperature through the evolution of CO and
and loses weight beyond this temperature through the evolution of CO and  . However, the XPS and FTIR data indicate that rinsing washes away most of the electrolyte residue. Therefore, the LiBOB data peaks are not likely to be from the salt, but rather from various oligomers, trigonal borates, or organoborate compounds in the SEI. The peak centered near
. However, the XPS and FTIR data indicate that rinsing washes away most of the electrolyte residue. Therefore, the LiBOB data peaks are not likely to be from the salt, but rather from various oligomers, trigonal borates, or organoborate compounds in the SEI. The peak centered near  may have minor contributions from the residual salt solvates. The
may have minor contributions from the residual salt solvates. The  sample data show several small peaks between 250 and
sample data show several small peaks between 250 and  , possibly resulting from the decomposition of various oligomers, and trigonal borate or organoborate compounds. There may be minor contributions from the electrolyte residue because the
, possibly resulting from the decomposition of various oligomers, and trigonal borate or organoborate compounds. There may be minor contributions from the electrolyte residue because the  salt is known to decompose above
salt is known to decompose above  . Distinct peaks are not seen in the
. Distinct peaks are not seen in the  sample data, which suggests low concentrations of organic species in the rinsed-electrode SEI. The small peaks observed in the
sample data, which suggests low concentrations of organic species in the rinsed-electrode SEI. The small peaks observed in the  sample data between 225 and
sample data between 225 and  may be associated with alkyl phosphates on the graphite surface.
may be associated with alkyl phosphates on the graphite surface.
Figure 14. The derivative of the weight change data (shown in Fig. 13) as a function of temperature. Peaks in the data are associated with the decomposition of SEI constituents.
Discussion
It is apparent from the data that there are differences between the constituents of the graphite SEI formed in various electrolytes, which indicates differences in the mechanisms of SEI formation. All SEIs contain constituents that are soluble in EMC and other constituents that are insoluble in EMC. Although most soluble components are precipitated salt-solvent complexes, some of the SEI constituents are also soluble in EMC. Many of the peaks associated with the soluble SEI constituents are structurally similar to previously reported solvent reduction products, such as lithium alkoxides and lithium alkyl carbonates. Proposed mechanisms of formation for these soluble compounds have been described previously.40, 41 The spectral data of the insoluble components of the SEI are very different for the different electrolytes indicating the presence of different compounds and, hence, different formation mechanisms.
The formation mechanisms also have to account for observations on graphite electrodes harvested from cells that underwent only formation cycling.42 The harvested graphite electrodes were rinsed in EMC, incorporated into new cells, and cycled with a lithium counter electrode. These cells did not show the electrolyte reduction peaks in the differential capacity plots ( vs
vs  ) that are observed during the first graphite lithiation cycle. Because EMC rinsing washes away a portion of the SEI layer (along with the electrolyte residue), it is apparent that the EMC-soluble SEI does not passivate the graphite surface. Rather, the SEI remaining after washing (i.e., the EMC-insoluble SEI) inhibits further electrolyte reduction on the graphite probably by modifying∕functionalizing the graphite edge planes.
) that are observed during the first graphite lithiation cycle. Because EMC rinsing washes away a portion of the SEI layer (along with the electrolyte residue), it is apparent that the EMC-soluble SEI does not passivate the graphite surface. Rather, the SEI remaining after washing (i.e., the EMC-insoluble SEI) inhibits further electrolyte reduction on the graphite probably by modifying∕functionalizing the graphite edge planes.
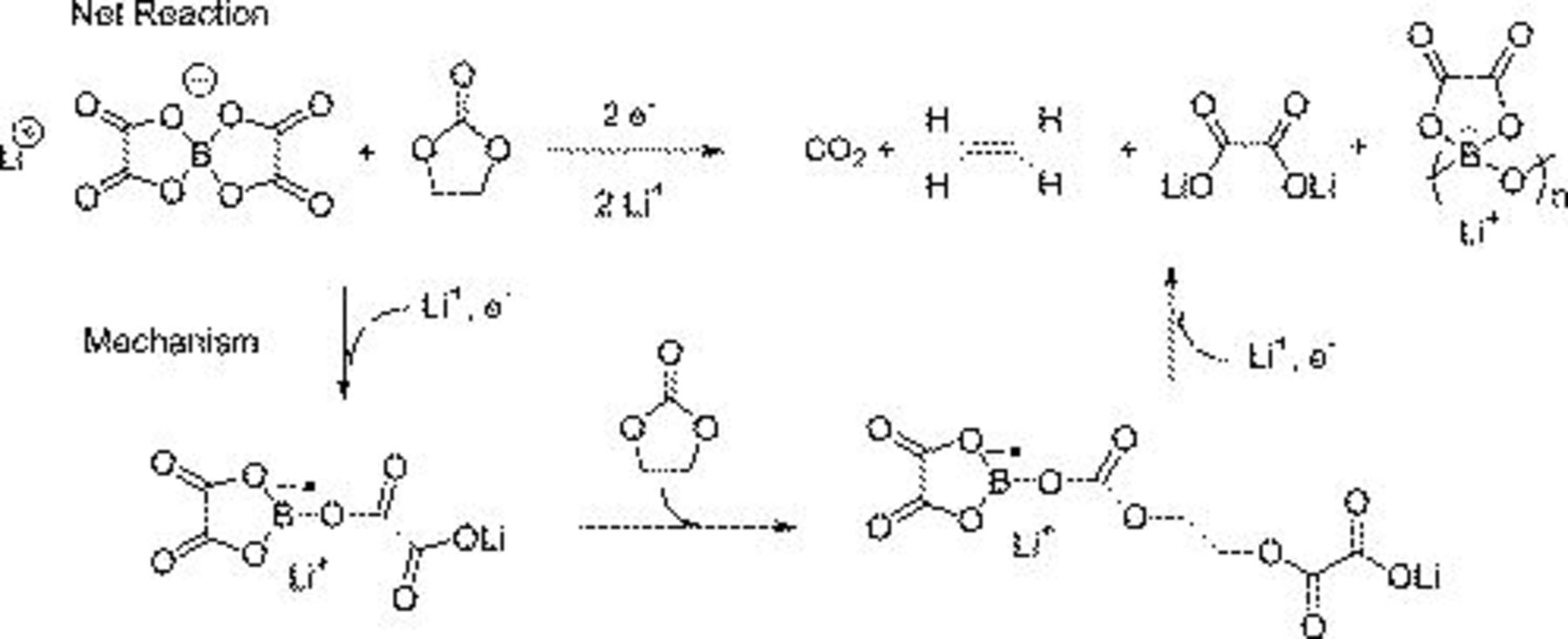
Typically, the SEI is attributed to the solvent reduction at the graphite surface. However, our various observations indicate that electrolyte salt reduction must also play a major role in SEI formation.25–29, 40 We propose the following SEI formation mechanisms that show the interactions between salt and solvent components during electrolyte reduction to explain our various data (see Fig. 15, 16, 17 and 18).
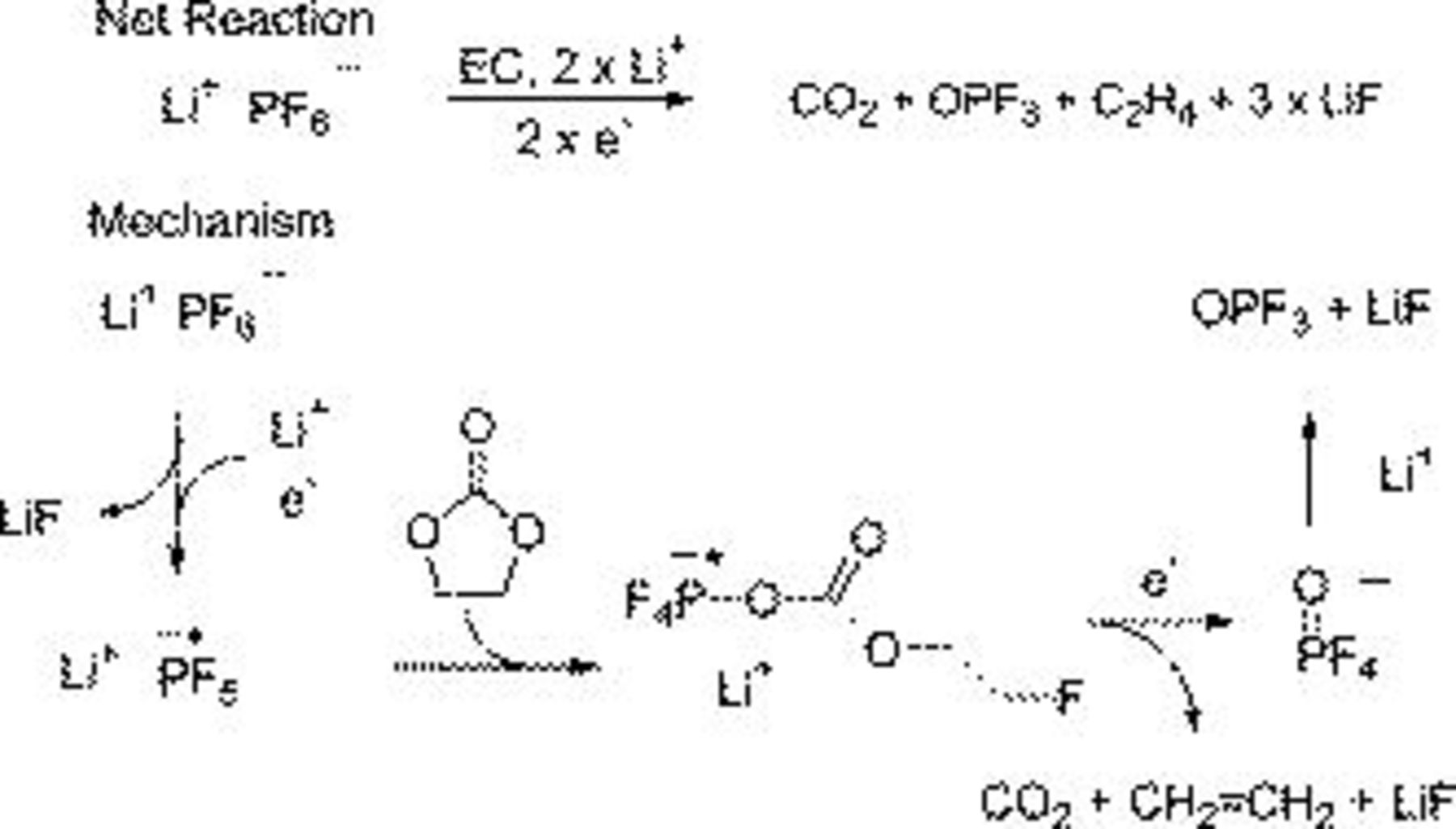
For  electrolytes the reduction of
electrolytes the reduction of  coupled with a ring-opening reaction of EC allows for the generation of
coupled with a ring-opening reaction of EC allows for the generation of  ,
,  , ethylene, and LiF (Fig. 15). The LiF precipitates while the
, ethylene, and LiF (Fig. 15). The LiF precipitates while the  increases the internal pressure of the cell. The
increases the internal pressure of the cell. The  reacts further with the carbonate solvents to generate related
reacts further with the carbonate solvents to generate related  and
and  while the ethylene is polymerized to generate polyethylene.43 For
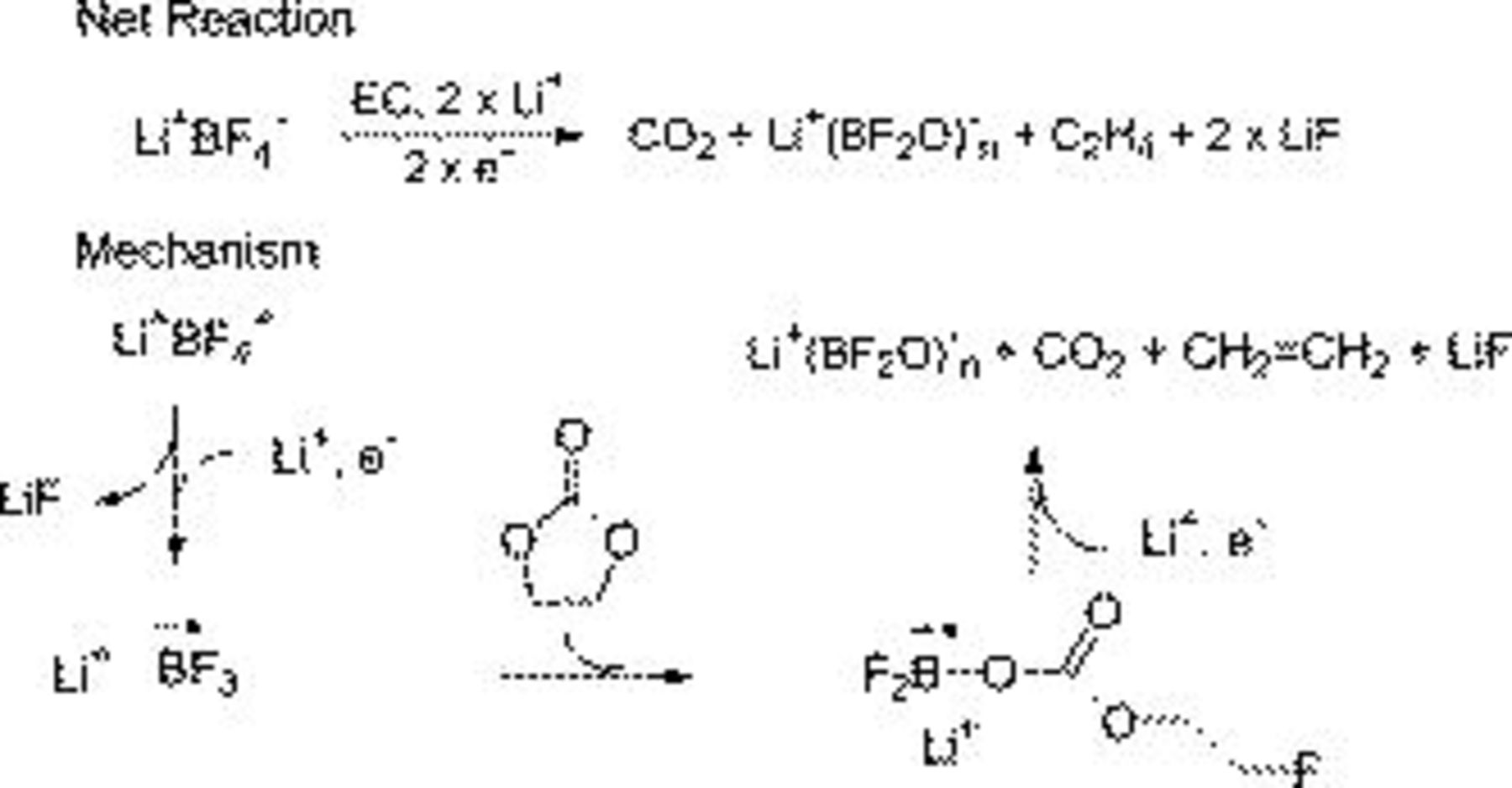
while the ethylene is polymerized to generate polyethylene.43 For  electrolytes the reduction of
electrolytes the reduction of  coupled with a ring-opening reaction of EC will be different since
coupled with a ring-opening reaction of EC will be different since  species are not stable. Therefore, the reaction forms
species are not stable. Therefore, the reaction forms  linear polymeric structures along with
linear polymeric structures along with  , ethylene, and LiF (Fig. 16). These polymeric structures could also have significant cross-linking via loss of the remaining fluorine∕oxygen exchange reaction on boron.
, ethylene, and LiF (Fig. 16). These polymeric structures could also have significant cross-linking via loss of the remaining fluorine∕oxygen exchange reaction on boron.
Figure 15. Reaction mechanism postulated to explain the formation of SEI constituents in the  electrolyte.
electrolyte.
Figure 16. Reaction mechanism postulated to explain the formation of SEI constituents in the  electrolyte.
electrolyte.
The reduction of  induces a ring-opening reaction of the salt, which coupled with a ring-opening reaction of EC generates a
induces a ring-opening reaction of the salt, which coupled with a ring-opening reaction of EC generates a  polymer and
polymer and  (Fig. 17a); an alternative reaction route generates the linear borate with an oxalate ligand on B and LiF (Fig. 17b). The presence of LiF in the XPS data suggests that Fig. 16b is a more likely reaction route. Partial ring opening of the LiBOB molecule on reduction coupled with EC ring-opening reaction generates a linear borate with an oxalate ligand and
(Fig. 17a); an alternative reaction route generates the linear borate with an oxalate ligand on B and LiF (Fig. 17b). The presence of LiF in the XPS data suggests that Fig. 16b is a more likely reaction route. Partial ring opening of the LiBOB molecule on reduction coupled with EC ring-opening reaction generates a linear borate with an oxalate ligand and  (Fig. 18). In addition, one would expect to observe additional reactions resulting from the ring opening of the oxalate ligand.
(Fig. 18). In addition, one would expect to observe additional reactions resulting from the ring opening of the oxalate ligand.
Figure 17. Reaction mechanisms postulated to explain the formation of SEI constituents in the  electrolyte.
electrolyte.
Figure 18. Reaction mechanism postulated to explain the formation of SEI constituents in the LiBOB electrolyte.
The observed impedance differences between graphite formed in various electrolytes, and attributed to differences in SEI morphology and composition,21 can be at least partially explained by our proposed mechanisms. The lower graphite impedance in  electrolytes may be attributed to the generation of
electrolytes may be attributed to the generation of  species, which may be of lower molecular weight and less likely to be cross-linked. However, these species may also be more soluble in the electrolyte, which explains the poorer SEI stability during elevated temperature aging
species, which may be of lower molecular weight and less likely to be cross-linked. However, these species may also be more soluble in the electrolyte, which explains the poorer SEI stability during elevated temperature aging  . The higher graphite impedance in
. The higher graphite impedance in  electrolytes may be due to the presence of a dense linear
electrolytes may be due to the presence of a dense linear  inorganic polymer on the graphite surface. However, this polymer is more stable at elevated temperatures, and its presence may explain the better performance retention of electrolytes containing
inorganic polymer on the graphite surface. However, this polymer is more stable at elevated temperatures, and its presence may explain the better performance retention of electrolytes containing  during elevated temperature aging. The
during elevated temperature aging. The  electrolyte can generate different types of borate polymers; the unique combination of these polymers and their apparently good lithium-ion conductivity may explain the low graphite impedance. The LiBOB electrolyte apparently generates a highly crosslinked borate∕oxalate SEI that has high impedance. It should be noted that the electrode impedance may also depend on the location of the inorganic products, such as LiF and
electrolyte can generate different types of borate polymers; the unique combination of these polymers and their apparently good lithium-ion conductivity may explain the low graphite impedance. The LiBOB electrolyte apparently generates a highly crosslinked borate∕oxalate SEI that has high impedance. It should be noted that the electrode impedance may also depend on the location of the inorganic products, such as LiF and  in the graphite SEI. These inorganic compounds can hinder lithium-ion motion when present in the "inner" SEI, adjacent to the graphite edge planes. Additional experiments are in progress to examine the validity of the various proposed mechanisms and their relationship to electrode impedance.
in the graphite SEI. These inorganic compounds can hinder lithium-ion motion when present in the "inner" SEI, adjacent to the graphite edge planes. Additional experiments are in progress to examine the validity of the various proposed mechanisms and their relationship to electrode impedance.
Conclusions
BF graphite electrodes produced by electrophoretic deposition and formed in electrolytes containing EC:EMC (3:7) solvent and various salts (
 ,
, 
 ,
, 
 , and
, and  LiBOB) were analyzed by FTIR, XPS, and TGA. Our data indicate the following:
LiBOB) were analyzed by FTIR, XPS, and TGA. Our data indicate the following:
- (1)Electrolyte reduction occurs on the graphite surface, but only during the first lithiation cycle. For graphite samples lithiated in LiBOB and
 electrolytes, reduction processes at multiple voltages (
electrolytes, reduction processes at multiple voltages ( and
and  vs Li), whereas samples lithiated in
vs Li), whereas samples lithiated in  and
and  electrolytes typically display electrolyte reduction at
electrolytes typically display electrolyte reduction at  vs Li.
vs Li. - (2)LiF and phosphate compounds, such as lithium alkyl phosphates
 (
( , etc.), and lithium oxyfluorophosphates
, etc.), and lithium oxyfluorophosphates  are important components of the graphite SEI formed in the
are important components of the graphite SEI formed in the  electrolyte.
electrolyte. - (3)LiF, alkyl borates, such as
 , lithium borates
, lithium borates  , and lithium fluoroborates
, and lithium fluoroborates  are prominent in the graphite SEI formed in the
are prominent in the graphite SEI formed in the  electrolyte. The reduction products of the
electrolyte. The reduction products of the  salt are less soluble in EMC than the related reduction products of
salt are less soluble in EMC than the related reduction products of  . The insoluble B-containing species may include inorganic∕polymer compounds (–B–O–) with or without cross-linking.
. The insoluble B-containing species may include inorganic∕polymer compounds (–B–O–) with or without cross-linking. - (4)A significant quantity of LiF,
 , and lithium oxalate (or alkyl esters of oxalic acid) is present in the SEI of graphite formed in
, and lithium oxalate (or alkyl esters of oxalic acid) is present in the SEI of graphite formed in  electrolyte. Our data also support the presence of organic species∕oligomeric compounds that decompose at elevated temperatures.
electrolyte. Our data also support the presence of organic species∕oligomeric compounds that decompose at elevated temperatures. - (5)Lithium oxalate (or alkyl esters of oxalic acid),
 , tri-coordinated borates and various oligomeric compounds are present in the graphite SEI formed in LiBOB. These compounds appear to be insoluble in EMC and decompose at temperatures above
, tri-coordinated borates and various oligomeric compounds are present in the graphite SEI formed in LiBOB. These compounds appear to be insoluble in EMC and decompose at temperatures above  . The graphite SEI may be fairly thick because graphite peaks are not observed in the XPS data and abundant organic decomposition products are found in the TGA data.
. The graphite SEI may be fairly thick because graphite peaks are not observed in the XPS data and abundant organic decomposition products are found in the TGA data. - (6)Lithium alkoxides, such as
 , and possibly small amounts of lithium alkyl carbonates, such as lithium diethylene carbonate, may also be present in the graphite SEI, but these species are washed off during electrode rinsing. Our data does not support the presence of
, and possibly small amounts of lithium alkyl carbonates, such as lithium diethylene carbonate, may also be present in the graphite SEI, but these species are washed off during electrode rinsing. Our data does not support the presence of  in any of the graphite SEI.
in any of the graphite SEI.
Acknowledgments
This work was supported by the U.S. Department of Energy (DOE), Office of Vehicle Technologies. We are grateful for the support of Tien Duong and Dave Howell at DOE, and Gary Henriksen and Dennis Dees at Argonne. We acknowledge the assistance of Magdalena Furczon (Argonne) for electrochemical measurements on the BF graphite samples and Liu Zhou (URI) for preparation and characterization of  .
.
Argonne National Laboratory assisted in meeting the publication costs of this article.