Abstract
Electrolytic studies on fused mixtures of ethyl pyridinium bromide and metallic chlorides have shown that the following metals can be deposited on the cathode: Ag, Cu, Bi, Pb, Sn, Ni, Co, Cd, Fe, Zn, and Al. Tests involving metallic sulfates and nitrates, as well as mixtures employing fused benzyl pyridinium bromide, showed that these metals can be deposited except in cases where nonionic complex compounds are formed.
In more extensive studies of the properties of aluminum chloride dissolved in fused ethyl pyridinium bromide it was found from melting‐point data that a compound having the composition  is formed, and that a very low‐melting eutectic (−40°C) occurs at 67 mole per cent
is formed, and that a very low‐melting eutectic (−40°C) occurs at 67 mole per cent  . Aluminum could be deposited from mixtures in the composition range 54–70 mole per cent
. Aluminum could be deposited from mixtures in the composition range 54–70 mole per cent 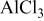 . Smooth plating of aluminum on iron, steel, copper, bronze, brass, platinum, lead, and tin could be accomplished by using an aluminum anode. The best plates were obtained when the composition of the bath was in the immediate neighborhood of the eutectic composition and at a temperature of 125°C, using a current density of 0.5 amp/dm2. Similar results were obtained using
. Smooth plating of aluminum on iron, steel, copper, bronze, brass, platinum, lead, and tin could be accomplished by using an aluminum anode. The best plates were obtained when the composition of the bath was in the immediate neighborhood of the eutectic composition and at a temperature of 125°C, using a current density of 0.5 amp/dm2. Similar results were obtained using  and ethylene dipyridinium dibromide mixtures.
and ethylene dipyridinium dibromide mixtures.
The state of ionization of the compound  in benzene solution was investigated by means of migration and conductivity experiments.
in benzene solution was investigated by means of migration and conductivity experiments.
