Abstract
 (R3m;
(R3m;  in hexagonal setting) was prepared and examined in nonaqueous lithium cells using 1M
in hexagonal setting) was prepared and examined in nonaqueous lithium cells using 1M 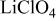 propylene carbonate solution at 30°C. The oxidation of
propylene carbonate solution at 30°C. The oxidation of  and the reduction of
and the reduction of  proceeded reversibly in the voltage region above 3.9 V. X‐ray diffraction (XRD) examinations indicated the reaction proceeded in a topotactic manner, i.e., two‐phase reactions
proceeded reversibly in the voltage region above 3.9 V. X‐ray diffraction (XRD) examinations indicated the reaction proceeded in a topotactic manner, i.e., two‐phase reactions  and a single‐phase reaction
and a single‐phase reaction  . A monoclinic phase
. A monoclinic phase  was observed in
was observed in  in addition to that at about
in addition to that at about 
 . Detailed open‐circuit voltage measurements were carried out. The open‐circuit voltage are invariable at 3.92 V for
. Detailed open‐circuit voltage measurements were carried out. The open‐circuit voltage are invariable at 3.92 V for  and at 4.50 V for
and at 4.50 V for  . The two straight lines are connected smoothly by a composition‐dependent curve for
. The two straight lines are connected smoothly by a composition‐dependent curve for  , which was consistent with the XRD observations. The differences and similarities between the solid‐state redox reactions of
, which was consistent with the XRD observations. The differences and similarities between the solid‐state redox reactions of  and
and  were discussed by comparing the structural and electrochemical data. Possible lithium ordering at
were discussed by comparing the structural and electrochemical data. Possible lithium ordering at  for this type of material is described in terms of a
for this type of material is described in terms of a  superlattice in a triangular lattice of sites.
superlattice in a triangular lattice of sites.
