Abstract
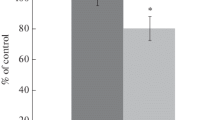
The success of studies on the neuroprotective properties of compounds depends on the used model of diseases of the central nervous system on animals. The approaches widely used to model cerebral ischemia/reperfusion were developed on young animals and may not be as efficient or may not be suitable at all for modeling on old animals, which are recommended for preclinical trials. The goal of the study was to detect age-related features of the effect of cerebral reperfusion of different duration (1 and 3 h) after two-vessel forebrain ischemia on the level of lipid peroxidation (LPO) products and the activity of Na+/K+-ATPase in the cerebral cortex of rats aged 22–24 months. We found a late accumulation (3 h, but not 1 h, after restoration of the blood flow) of LPO products, specifically, triene conjugates and Schiff bases, and a decrease in Na+/K+-ATPase activity in the cerebral cortex of old rats as compared with young animals. This indicates the features of the molecular and physiological mechanisms of the development of disorders in the brain during ischemia/reperfusion in old and young animals. It is necessary to take into account the identified differences in these mechanisms in the development and testing of compounds that will be subsequently used for the treatment of elderly patients with stroke and ischemic brain damage.

Similar content being viewed by others
REFERENCES
Gavrilov, V.B., Gavrilova, A.R., and Mazhul’, L.M., Determination of lipid peroxidation products in blood serum according to the test with thiobarbituric acid, Vopr. Med. Khim., 1987, no. 1, pp. 118–122.
Zorina, I.I., Zakharova, I.O., Bayunova, L.V., and Avrova, N.F., Insulin administration prevents accumulation of conjugated dienes and trienes and inactivation of Na+, K+-ATPase in the rat cerebral cortex during two-vessel forebrain ischemia and reperfusion, J. Evol. Biochem. Physiol., 2018, vol. 54, no. 3, pp. 246–249.
Zorina, I.I., Galkina, O.V., Bayunova, L.V., and Zakharova, I.O., Effect of insulin on lipid peroxidation and glutathione levels in a two-vessel occlusion model of rat forebrain ischemia followed by reperfusion, J. Evol. Biochem. Physiol., 2019, vol. 55, no. 4, pp. 333–335. https://doi.org/10.1134/S0022093019040094
Molchanova, S.M., Moskvin, A.N., Zakharova, I.Yu., et al., Effects of two-vessel forebrain ischemia and of administration of indomethacin and quinacrine on Na+, K+-ATPase activity in various rat brain areas, J. Evol. Biochem. Physiol., 2005, vol. 41, no. 1, pp. 39–46.
Shvedova, A.A. and Polyanskii, N.B., Determination of lipid hydroperoxides conjugates in tissue extracts, in Issledovanie sinteticheskikh i prirodnykh antioksidantov in vivo i in vitro (Synthetic and Natural Antioxidants in Vivo and in Vitro), Moscow: Nauka, 1992, pp. 74–75.
Anuncibay-Soto, B., Pérez-Rodríguez, D., Llorente, I.L., et al., Age-dependent modifications in vascular adhesion molecules and apoptosis after 48-h reperfusion in a rat global cerebral ischemia model, Age, 2014, vol. 36, no. 5, p. 9703. https://doi.org/10.1007/s11357-014-9703-7
Attwell, D. and Laughlin, S.B., An energy budget for signaling in the grey matter of the brain, J. Cereb. Blood Flow Metab., 2001, vol. 21, no. 10, pp. 1133–1145. https://doi.org/10.1097/00004647-200110000-00001
Badan, I., Buchhold, B., Hamm, A., et al., Accelerated glial reactivity to stroke in aged rats correlates with reduced functional recovery, J. Cereb. Blood Flow Metab., 2003, vol. 23, no. 7, pp. 845–854. https://doi.org/10.1097/01.WCB.0000071883.63724.A7
Bidlack, W.R. and Tappel, A.L., Fluorescent products of phospholipids during lipid peroxidation, Lipids, 1973, vol. 8, no. 4, pp. 203–207.
Boveris, A. and Navarro, A., Brain mitochondrial dysfunction in aging, IUBMB Life, 2008, vol. 60, no. 5, pp. 308–314. https://doi.org/10.1002/iub.46
Broughton, B.R.S., Reutens, D.C., and Sobey, C.G., Apoptotic mechanisms after cerebral ischemia, Stroke, 2009, vol. 40, no. 5, pp. e331–e339. https://doi.org/10.1161/STROKEAHA.108.531632
Folch, J., Lees, M., and Sloane Stanley, G.H., A simple method for the isolation and purification of total lipides from animal tissues, J. Biol. Chem., 1957, vol. 226, no. 1, pp. 497–509.
Fraser, C.L. and Arieff, A.I., Na-K-ATPase activity decreases with aging in female rat brain synaptosomes, Am. J. Physiol.: Renal Physiol., 2001, vol. 281, no. 4, pp. F674–F678. https://doi.org/10.1152/ajprenal.2001.281.4.F674
Gibson, G.E., Starkov, A., Blass, J.P., et al., Cause and consequence: mitochondrial dysfunction initiates and propagates neuronal dysfunction, neuronal death and behavioral abnormalities in age-associated neurodegenerative diseases, Biochim. Biophys. Acta,Mol. Basis Dis., 2010, vol. 1802, pp. 122–134. https://doi.org/10.1016/j.bbadis.2009.08.010
Lorenzini, P., Pestalozza, S., Fortuna, S., et al., Transient global cerebral hypoxia-ischemia in rats: age-related differences in neuronal damage and responsiveness of metabotropic glutamate receptors to excitatory amino acid agonists, Fund. Clin. Pharmacol., 1996, vol. 10, p. 199.
Moretti, A., Ferrari, F., and Villa, R.F., Neuroprotection for ischemic stroke: current status and challenges, Pharmacol. Ther., 2015, vol. 146, pp. 23–34. https://doi.org/10.1016/j.pharmthera.2014.09.003
Moskowitz, M.A., Grotta, J.C., and Koroshetz, W.J., The NINDS stroke progress review group final analysis and recommendations, Stroke, 2013, vol. 44, no. 8, pp. 2343–2350. https://doi.org/10.1161/STROKEAHA.113.001192
Müldner, A. and Hoyer, S., Delayed decrease of excitatory amino acid neurotransmitters in parietotemporal cortex and hippocampus after complete cerebral ischemia in aged rats, Arch. Gerontol. Geriatr., 1997, vol. 24, no. 1, pp. 23–33.
Navarro, A. and Boveris, A., The mitochondrial energy transduction system and the aging process, Am. J. Physiol.: Cell Physiol., 2007, vol. 292, no. 2, pp. C670–C686. https://doi.org/10.1152/ajpcell.00213.2006
Navarro, A. and Boveris, A., Mitochondrial nitric oxide synthase, mitochondrial brain dysfunction in aging, and mitochondria-targeted antioxidants, Adv. Drug Delivery Rev., 2008, vol. 60, nos. 13–14, pp. 1534–1544. https://doi.org/10.1016/j.addr.2008.05.002
Petcu, E.B., Sfredel, V., Platt, D., et al., Cellular and molecular events underlying the dysregulated response of the aged brain to stroke: a mini-review, Gerontology, 2008, vol. 54, no. 1, pp. 6–17. https://doi.org/10.1159/000112845
Petit-Taboue, M.C., Landeau, B., Desson, J.F., et al., Effects of healthy aging on the regional cerebral metabolic rate of glucose assessed with statistical parametric mapping, NeuroImage, 1998, vol. 7, no. 3, pp. 176–184.
Philip, M., Benatar, M., Fisher, M., and Savitz, S.I., Methodological quality of animal studies of neuroprotective agents currently in phase II/III acute ischemic stroke trials, Stroke, 2009, vol. 40, no. 2, pp. 577–581. https://doi.org/10.1161/STROKEAHA.108.524330
Popa-Wagner, A., Badan, I., Walker, L., et al., Accelerated infarct development, cytogenesis and apoptosis following transient cerebral ischemia in aged rats, Acta Neuropathol., 2007, vol. 113, no. 3, pp. 277–293. https://doi.org/10.1007/s00401-006-0164-7
Raval, A.P., Liu, C., and Hu, B.R., Rat model of global cerebral ischemia: the two-vessel occlusion (2VO) model of forebrain ischemia, in Animal Models Acute Neurological Injuries, New York: Springer-Verlag, 2009, pp. 77–86. https://doi.org/10.1007/978-1-60327-185-1_7
Siems, W.G., Hapner, S.J., and van Kuijk, F.J., 4-Hydroxynonenal inhibits Na+-K+-ATPase, Free Radical Biol. Med., 1996, vol. 20, no. 2, pp. 215–223. https://doi.org/10.1016/0891-5849(95)02041-1
Simã o, F., Matté, A., Matté, C., et al., Resveratrol prevents oxidative stress and inhibition of Na+K+-ATPase activity induced by transient global cerebral ischemia in rats, J. Nutr. Biochem., 2011, vol. 22, no. 10, pp. 921–928. https://doi.org/10.1016/j.jnutbio.2010.07.013
Srikanthan, K., Shapiro, J., and Sodhi, K., The role of Na/KATPase signaling in oxidative stress related to obesity and cardiovascular disease, Molecules, 2016, vol. 21, no. 9, p. 1172. https://doi.org/10.3390/molecules21091172
Sutherland, G.R., Dix, G.A., and Auer, R.N., Effect of age in rodent models of focal and forebrain ischemia, Stroke, 1996, vol. 27, no. 9, pp. 1663–1667. https://doi.org/10.1161/01.STR.27.9.1663
Yager, J.Y., Shuaib, A., and Thornhill, J., The effect of age on susceptibility to brain damage in a model of global hemispheric hypoxia-ischemia, Brain Res. Dev. Brain Res., 1996, vol. 93, nos. 1–2, pp. 143–154. https://doi.org/10.1016/0165-3806(96)00026-0
Yu, W.H., Go, L., Guinn, B.A., et al., Phenotypic and functional changes in glial cells as a function of age, Neurobiol. Aging, 2002, vol. 23, no. 1, pp. 105–115. https://doi.org/10.1016/S0197-4580(01)00258-5
Funding
This work was supported by the Russian Foundation for Basic Research (project no. 18-315-00285) and partially by the state tasks of the Federal Agency for Scientific Organizations of Russia (topic no. AAAA-A18-118012290427-7).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflict of interest.
Statement on animal welfare. All experiments were conducted in strict accordance with the regulations developed and approved by the Ethics Committee of the Sechenov Institute of Evolutionary Physiology and Biochemistry, Russian Academy of Sciences, regulations and requirements set out in the documents “European Communities Council Directive 1986” (86/609/EEC) and “Guide for the Care and Use of Laboratory Animals.”
Additional information
Translated by A. Barkhash
Rights and permissions
About this article
Cite this article
Zorina, I.I., Fokina, E.A., Zakharova, I.O. et al. Characteristics of Changes in Lipid Peroxidation and Na+/K+-ATPase Activity in the Cortex of Old Rats in Conditions of Two-Vessel Cerebral Ischemia/Reperfusion. Adv Gerontol 10, 156–161 (2020). https://doi.org/10.1134/S2079057020020162
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2079057020020162