Abstract
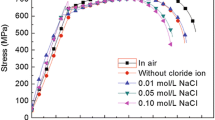
Stress corrosion cracking behavior of API 5L X65 steel in a NS4 solution with different pH was investigated by electrochemical impedance under slow strain rate tensile test. NS4 solution was selected as corrosive electrolyte under disbonded pipeline coatings. According to the analysis of tensile test, steel was susceptible to SCC in a NS4 solution. The reduction area ratio in the air was measured to be 70.71%, which was more significant than the reduction obtained in a NS4 solution with different pH. EIS in different strain time in acidic solution showed perfect semicircles, which indicated high dissolution rate and some hydrogen penetration effect in this environment. EIS in neutral and basic NS4 solution presented effective constant phase elements in the transmission line model for the porous electrode, which indicated SCC behavior of steel in these solutions. Phase angle analysis indicated that cracks grow considerably after 12 h. Besides, SEM illustrated a ductile type of fracture in air and brittle type of fracture in all NS4 solution.


















Similar content being viewed by others
REFERENCES
Sotelo-Mazon, O., Valdez, S., Porcayo-Calderon, J., Henao, J., Cuevas-Arteaga, C., Poblano-Salas, C.A., and Martinez-Gomez, L., Prot. Met. Phys. Chem. Surf., 2020, vol. 56, pp. 427–437.
Ghiasvand, M., Zaarei, D., Danaee, I., Mogoie, B., and Salehi Nasab, H., Prot. Met. Phys. Chem. Surf., 2019, vol. 55, pp. 1154–1160.
Petrov, N.N., Alovyagina, A.S., Mikheev, M.N., Bukov, N.N., and Panyushkin, V.T., Prot. Met. Phys. Chem. Surf., 2020, vol. 56, pp. 603–608.
Petrunin, M.A., Maksaeva, L.B., Rybkin, A.A., Gladkikh, N.A., Yurasova, T.A., Maleeva, M.A., and Marshakov, A.I., Prot. Met. Phys. Chem. Surf., 2019, vol. 55, pp. 1335–1340.
Srinivasa Rao, G., Srinivasa Rao, K., Srinivasa Rao, P., Koteswara Rao, S.R., and Madhusudan Reddy, G., Prot. Met. Phys. Chem. Surf., 2018, vol. 54, pp. 866–875.
Maleeva, M.A., Petrunin, M.A., Maksaeva, L.B., Yurasova, T.A., and Marshakov, A.I., Prot. Met. Phys. Chem. Surf., 2016, vol. 52, pp. 1107–1113.
Pourazizi, R., Mohtadi-Bonab, M.A., and Szpunar, J.A., Eng. Failure Anal., 2020, vol. 109, p. 104400.
Maocheng, Y.A., Jin, X.U., Libao, Y.U., Tangqing, W.U., Cheng, S.U., and Wei, K.E., Corros. Sci., 2016, vol. 110, pp. 23–34.
Wang, L., Xin, J., Cheng, L., Zhao, K., Sun, B., Li, J., Wang, X., and Cui, Z., Corros. Sci., 2019, vol. 147, pp. 108–127.
Nyrkova, L., Osadchuk, S., Melnichuk, S., Rybakov, A., Ostapyuk, S., and Borysenko, Y., Mater. Today: Proc., 2019, vol. 6, pp. 279–287.
Li, C.X., Dang, S.H., Bao, X.L., Zhang, P., Wang, G.Z., Long, X.J., and Han, P.D., Chin. J. Phys., 2019, vol. 59, pp. 242–249.
Liu, Z.Y., Li, X.G., Du, C.W., Zhai, G.L., and Cheng, Y.F., Corros. Sci., 2008, vol. 50, pp. 2251–2257.
Liu, Z.Y., Du, C.W., Zhang, X., Wang, F.M., and Li, X.G., Acta Metall. Sin. (Engl. Lett.), 2013, vol. 26, pp. 489–496.
Fang, B.Y., Atrens, A., Wang, J.Q., Han, E.H., Zhu, Z.Y., and Ke, W., J. Mater. Sci., 2003, vol. 38, pp. 127–132.
Zheng, X., Castaneda, H., Gao, H., and Srivastava, A., Corrosion, 2019, vol. 153, pp. 53–61.
Bosch, R.W., Corros. Sci., 2005, vol. 47, no. 1, pp. 125–143
Bosch, R.W., Moons, F., Zheng, J.H., and Bogaerts, W.F., Corrosion, 2001, vol. 57, pp. 532–539.
Lou, X. and Singh, P.M., Electrochim. Acta, 2011, vol. 56, pp. 1835–1847.
Barraza-Fierro, J.I., Serna-Barquera, S.A., Campillo-Illanes, B.F., and Castaneda, H., Metall. Mater. Trans. A, 2017, vol. 48, pp. 1944–1958.
Monnot, M., Roche, V., Estevez, R., Mantel, M., and Nogueira, R.P., Electrochim. Acta, 2017, vol. 252, pp. 58–66.
Danaee, I., Nikparsa, P., Khosravi-Nikou, M.R., Eskandari, H., and Nikmanesh, S., Prot. Met. Phys. Chem. Surf., 2019, vol. 55, pp. 1000–1013.
Havashinejadian, E., Danaee, I., Eskandari, H., and Nikmanesh, S., J. Electrochem. Sci. Technol., 2017, vol. 8, pp. 115–123.
Danaee, I. and Nikparsa, P., J. Mater. Eng. Perform., 2019, vol. 28, pp. 5088–5103.
Eloot, K., Debuyck, F., Moors, M., and Van Peteghem, A.P., J. Appl. Electrochem., 1995, vol. 25, pp. 326–333.
de Levie, R., Electrochim. Acta, 1963, vol. 8, pp. 751–780.
Bisquert, J., Phys. Chem. Chem. Phys., 2000, vol. 2, pp. 4185–4192.
Keiser, H., Beccu, K.D., and Gutjahr, M.A., Electrochim. Acta, 1976, vol. 21, pp. 539–543.
Bastidas, D.M., Corrosion, 2007, vol. 63, pp. 515–521.
Cooper, S.J., Bertei, A., Finegan, D.P., and Brandon, N.P., Electrochim. Acta, 2017, vol. 251, pp. 681–689.
Huang, J., Electrochim. Acta, 2018, vol. 281, pp. 170–188.
Oskuie, A.A., Shahrabi, T., Shahriari, A., and Saebnoori, E., Corros. Sci., 2012, vol. 61, pp. 111–122.
Contreras, A., Hernández, S.L., Orozco-Cruz, R., and Galvan-Martínez, R., Mater. Des., 2012, vol. 35, pp. 281–289.
Hardie, D., Charles, E.A., and Lopez, A.H., Corros. Sci., 2006, vol. 48, pp. 4378–4385.
Contreras, A., Salazar, M., Carmona, A., and Galván-Martínez, R., Mater. Res., 2017, vol. 20, pp. 1201–1210.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghobadi, M., Danaee, I., Saebnoori, E. et al. Impedance Studies on Stress Corrosion Cracking Behavior of Steel Pipeline in NS4 Solution under SSRT Test Condition. Prot Met Phys Chem Surf 57, 634–646 (2021). https://doi.org/10.1134/S2070205121030126
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205121030126