Abstract
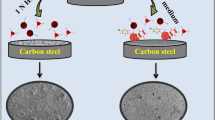
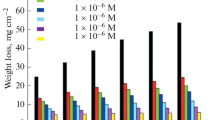
The corrosion inhibition characteristics of 2-mercapto benzimidazole (MBI), 2-mercapto benzimidazolyl-ethyl acetate (MBEA), 2-hydroxy benzimidazole (HBI) and 2-hydroxy 5-nitro benzimidazole (HNBI) on zinc corrosion in 0.1 M hydrochloric acid were investigated by weight loss and potentiodynamic polarization techniques. The inhibition efficiency increased with increase in inhibitor concentration but decreased with increase in temperature. The adsorption of MBI and MBEA obeyed Langmuir’s adsorption isotherm but HBI and HNBI followed Temkin’s adsorption isotherm. Thethermodynamic functions for adsorption processes were evaluated. The existence of film on metal surface in presence of inhibitors was established by scanning electron microscopy (SEM) images.
Similar content being viewed by others
References
Agrawal, Y.K., Talati, J.D., Shah, M.D., et al., Corrosion Sci., 2004, vol. 46, p. 651.
Bhajiwala, H.M. and Vashi, R.T., Bull. Electrochem., 2002, vol. 18, p. 261.
Abdel Wahab, I., Khalifa, O., and Mostafa Abo El Khair, B., Asian J. Chem., 1993, vol. 5, p. 1084.
Tamil Selvi, S., Raman, V., and Rajendran, N., J. Appl. Electrochem., 2003, vol. 33, p. 1175.
Rudresh, H.B. and Mayanna, S.M., Mater. and Corros., 2004, vol. 31, p. 286.
Stupnisek-Lisac, E. and Podbrscek, S., J. Appl. Electrochem., 1994, vol. 24, p. 779.
Emregul Kaan, C., Abdulkadir Akay, A., and Orhan Atakol, Mater. Chem. Phys., 2005, vol. 93, p. 325.
Morad, M.S., J. Appl. Electrochem., 1999, vol. 29, p. 619.
Ashassi-Sorkhabi, H., Shaabani, B., and Seifzadeh, D., Electrochem. Acta, 2005, vol. 50, p. 3446.
Abdallah, M., Corrosion Sci., 2002, vol. 44, p. 717.
Shanbhag, A.V., Prabhu, R.A., Kulkarni, G.M., et al., Indian J. Chem. Technol., 2007, vol. 14, p. 584.
Sahin, M., Bigic, S., and Yilmaz, H., Appl. Surf. Sci., 2002, vol. 195, p. 1.
Prabhu, R.A., Shanbhag, A.V., and Venkatesha, T.V., J. Appl. Electrochem., 2007, vol. 37, p. 491.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Shanbhag, A.V., Venkatesha, T.V. & Praveen, B.M. Benzimidazole derivatives as corrosion inhibitors for zinc in acid solution. Prot Met Phys Chem Surf 49, 587–590 (2013). https://doi.org/10.1134/S2070205113050146
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205113050146