Abstract
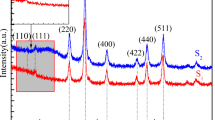
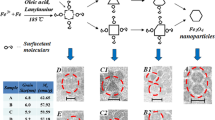
Magnetic nanoparticles (Fe3O4) were synthesized by the solvothermal method using FeCl3 · 6H2O and ethylene glycol as a reactant. Powder X-ray diffraction, FT-IR, TEM, SEM, and VSM were used to characterize the magnetic particles. The reacting factors, such as reacting time, the concentration of iron source and surfactant, especially the effect of NaAc · 3H2O, were studied. The results indicated that NaAc · 3H2O plays the role not only as a dispersant but also a structure-directing agent. The synthesized Fe3O4 particles showed excellent magnetic property, which made them have potential for application in magnetic nanodevices and biomedicine.
Similar content being viewed by others
References
García del Muro, M., Konstantinovic, Z., Varela, M., Batlle, X., and Labarta, A., Magnetic Properties of Co Nanoparticles in Zirconia Matrix, J. Magn. Magn. Mater., 2007, vol. 316, no 2, pp. 103–105.
Chen, J. and Peng, Z., Preparation and Properties of a Magnetic-Nanometer TiO2/Fe2O3 Composite Photocatalyst, Acta Chim. Sin. (Engl. Ed.), 2004, vol. 62, no. 20, pp. 2093–2097.
Maria Claesson, E., Mehendale, N.C., Gebbink, R.J.M.K., van Koten, G., and Philipse, A.P., Magnetic Silica Colloids for Catalysis, J. Magn. Magn. Mater., 2007, vol. 311, no. 1, pp. 41–45.
Pathmamanoharan, C. and Philipse, A.P., Preparation and Properties of Monodisperse Magnetic Cobalt Colloids Grafted with Polyisobutene, J. Colloid Sci., 1998, vol. 205, no. 2, pp. 340–353.
Dou, Y.H., Zhang, L., and Gu, H.C., Synthesis, Characterization, and Self-Assembly of Monodispersed Fe3O4 Nanoparticles, J. Funct. Mater., 2002, vol. 38, no. 1, pp. 119–122.
Sugimoto, T. and Matijevic, E., Formation of Uniform Spherical Magnetite Particles by Crystallization from Ferrous Hydroxide Gels, J. Colloid Interface Sci., 1980, vol. 74, pp. 227–243.
Daou, T.J., Pourroy, G., Begin-Colin, S., Greneche, J.M., Ulhaq-Bouillet, C., Legare, P., Bernhardt, P., Leuvrey, C., and Rogez, G., Hydrothermal Synthesis of Monodisperse Magnetite Nanoparticles, Chem. Mater., 2006, vol. 18, pp. 4399–4404.
Sugimoto, T. and Matijevic, E., Formation of Uniform Spherical Magnetite Particles by Crystallization from Ferrous Hydroxide Gels, J. Colloid Interface Sci., 1980, vol. 74, pp. 227–243.
Kang, Y.S., Risbud, S., Rabolt, J.F., and Stroeve, P., Synthesis and Characterization of Nanometer-Size Fe3O4 and γ-Fe2O3 Particles, Chem. Mater., 1996, vol. 8, pp. 2209–2211.
Fried, T., Shemer, G., and Markovich, G., Ordered Two-Dimensional Arrays of Ferrite Nanoparticles, J. Adv. Mater., 2001, vol. 13, pp. 1158–1161.
Wang, S., Xin, H., and Qian, Y., Preparation of Nanocrystalline Fe3O4 by γ-Ray Radiation, J. Mater. Lett., 1997, vol. 33, pp. 113–116.
Vollath, D. and Szabo, D.V., Synthesis and Magnetic Properties of Nanostructured Maghemite, J. Mater. Res., 1997, vol. 12, pp. 2175–2182.
Feldmann, C. and Jungk, H.O., Polyol-Mediated Preparation of Nanoscale Oxide Particles, J. Angew. Chem., Int. Ed., 2001, vol. 40, pp. 359–362.
Li, Z., Chen, H., Bao, H., and Gao, M., One-Pot Reaction to Synthesize Water-Soluble Magnetite Nanocrystals, Chem. Mater., 2004, vol. 16, no. 8, pp. 1391–1393.
Sun, S. and Zeng, H.J., Size-Controlled Synthesis of Magnetite Nanoparticles, J. Am. Chem. Soc., 2002, vol. 124, pp. 8204–8205.
Si, S., Li, C., Wang, X., Yu, D., Peng, Q., and Li, Y., Magnetic Monodisperse Fe3O4 Nanoparticles, Cryst. Growth Des., 2005, vol. 5, no. 2, pp. 391–393.
Pinna, N., Grancharov, S., Beato, P., Bonville, P., Antonietti, M., and Niederberger, M., Magnetite Nanocrystals: Nonaqueous Synthesis, Characterization, and Solubility, Chem. Mater., 2005, vol. 17, pp. 3044–3049.
Deng, H., Li, X., Peng, Q., Wang, X., Chen, J., and Li, Y., Monodisperse Magnetic Single-Crystal Ferrite Microspheres, J. Angew. Chem., Int. Ed., 2005, vol. 44, pp. 2782–2785.
Veliotti, J.B., New Research on Solid State Chemistry, New York: Nova Science, 2007, pp. 241–267.
Xiang, W.-D., Yang, Y.-X., Chen, J., Wang, Z., and Liu X.-N., Preparation of Mesoporous Silica Using Amphoteric Surfactant Potassium and Sodium N-Dodecyl Glycine Template, J. Am. Ceram. Soc., 2008, vol. 91, no. 5, pp. 1517–1521.
Pan, M.-C., Yang, Y.-X., Ying, H.-P., Jia, X.-C., Chen, Y.-R., and Tang, Y., Effect of Variant Counterions on Stability and Particle Size of Silica Sol, Chin. J. Chem., 2007, vol. 25, no. 10, pp. 1514–1521.
Guo, W., Sun, Y.W., Luo, G.S., and Wang, Y.J., Interaction of PEG with Ionic Surfactant SDS to Form Template for Mesoporous Material, J. Colloids Surf., 2005, vol. 252, pp. 71–77.
Kodama, R.H., Berkowitz, A.E., McNiff, E.J., and Foner, S., Surface Spin Disorder in NiFe2O4 Nanoparticles, Phys. Rev. Lett., 1996, vol. 77, pp. 394–397.
Meng, Z., Zhang, D.T., and Wang, C.P., Synthesis and Characterization of Magnetic Nanocrystal Fe3O4 in O2 Inducing and Air Slow Oxidation, J. Chin. Spectrosc. Lab. (Guangpu Shiyanshi), 2003, vol. 20, no. 4, pp. 489–491.
Wang, H., Xu, L.-H., Di, Y.-P., and Zhang, H., Preparation of Nanosized Magnetism Fe3O4 Powders by Reduction Co-Precipitation Method, Nanosci. Nanotechnol., 2007, vol. 12, no. 6, pp. 42–49.
Zhou, X.L. and Bi, H., Hydrothermal Synthesis and Characterization of Magnetic Fe3O4 Nanoparticles, J. Anhui Univ. Sci. Technol., Nat. Sci. Ed. (Anhui Ligong Daxue Xuebao, Ziran Kexueban), 2006, vol. 30, no. 2, pp. 75–80.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Huang, Y., Zhang, L., Huan, W. et al. A study on synthesis and properties of Fe3O4 nanoparticles by solvothermal method. Glass Phys Chem 36, 325–331 (2010). https://doi.org/10.1134/S1087659610030090
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1087659610030090