Abstract
The solid-phase reaction of [Pt(NH3)4]Cl2 and (NH4)6Mo7O24 under argon in the temperature range from 50 to 500°C was studied by thermal analysis and mass spectrometry. According to the X-ray powder diffraction, X-ray photoelectron spectroscopy, and elemental analysis data, the product consists of ordered Pt3Mo phase with an insignificant amount of molybdenum(VI) oxide. The described reaction is accompanied by reduction of the metals, which is promoted by Pt2+ ions in the presence of ammonia.
Similar content being viewed by others
Notes
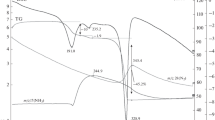
We failed to identify phase composition of the intermediate product obtained at 265°С.
The reactions on exposure to air were carried out in a tubular furnace on heating at a rate of 10 deg/min.
REFERENCES
Sankar, M., Dimitratos, N., Miedziak, P.J., Wells, P.P., Kiely, J., and Hutchings, G.J., Chem. Soc. Rev., 2012, vol. 41, p. 8099. https://doi.org/10.1039/c2cs35296f
Wei, Zh., Sun, J., Li, Y., Datye, A.K., and Wong, Y., Chem. Soc. Rev., 2012, vol. 41, p. 7994. https://doi.org/10.1039/c2cs35201j
Wang, D., Peng, Q., and Li, Y., Nano Res., 2010, no. 3, p. 574. https://doi.org/10.1007/s12274-010-0018-4
Lian, X., Guo, W., Liu, F., Yang, Y., Xiao, Y., Zhang, Y., and Tian, W.Q., Comput. Mater. Sci., 2015, vol. 96, part A, p. 237. https://doi.org/10.1016/j.commatsci.2014.09.025
Toyoda, T., Nishihara, Y., and Qian, E.W., Fuel Process. Technol., 2014, vol. 125, p. 86. https://doi.org/10.1016/j.fuproc.2014.03.033
Morán, C., González, E., Sánchez, J., Solano, R., Carruyo, G., and Moronta, A., J. Colloid Interface Sci., 2007, vol. 315, no. 1, p. 164. https://doi.org/10.1016/j.jcis.2007.06.027
Boufaden, N., Akkari, R., Pawelec, B., Fierro, J.L.G., Said, Z.M., and Ghorbel, A., J. Mol. Cat. A: Chem., 2016, vol. 420, p. 96. https://doi.org/10.1016/j.molcata.2016.04.011
Lu, G.-P., Ma, X.-B., Yang, H.-F., Kong, D.-S., and Feng, Y.-Y., Int. J. Hydrogen Energy, 2015, vol. 40, no. 17, p. 5889. https://doi.org/10.1016/j.ijhydene.2015.03.026
Hao, Y., Wang, X., Zheng, Y., Shen, J., Yuan, J., Wang, A.-j., Niu, L., and Huang, S., Electrochim. Acta, 2016, vol. 198, p. 127. https://doi.org/10.1016/j.electacta.2016.03.054
Dietrich, P.J., Sollberger, F.G., Akatay, M.C., Stach, E.A., Delgass, W.N., Miller, J.T., and Ribeiro, F.H., Appl. Catal., B, 2014, vol. 156, p. 236. https://doi.org/10.1016/j.apcatb.2014.03.016
Zhang, H., Wang, Z., Li, S., Jiao, Y., Wang, J., Zhu, Q., and Li, X., Appl. Therm. Eng., 2017, vol. 111, p. 811. https://doi.org/10.1016/j.applthermaleng.2016.10.006
Fesik, E.V, Buslaeva, T.M., Melnikova, T.I., and Tarasova, L.S., Inorg. Mater., 2017, vol. 53, no. 10, p. 1033. https://doi.org/10.1134/S0020168517100065
Zarazhevskii, V.I., Grebnev, V.V., Fesik, E.V., and Mal’chikov, G.D., Vestn. MITKhT, 2010, vol. 5, no. 6, p. 70.
Sintez kompleksnykh soedinenii metallov platinovoi gruppy (Synthesis of Coordination Compounds of Platinum Group Metals), Chernyaev, I.I., Ed., Moscow: Nauka, 1964.
Boultif, A. and Louer, D., J. Appl. Crystallogr., 2004, vol. 37, p. 724. https://doi.org/10.1107/S0021889804014876
Blagorodnye metally (Noble Metals), Savitskii, E.M., Ed., Moscow: Metallurgiya, 1984.
Raddi de Araujo, L.R. and Schmal, M., Appl. Catal., A, 2000, vol. 203, no. 2, p. 275. https://doi.org/10.1016/S0926-860X(00)00487-7
Kim, J.-S., Seol, D., Ji, J., Jang, H.-S., Kim, Y., and Lee, B.-J., Calphad, 2017, vol. 59, p. 131. https://doi.org/10.1016/j.calphad.2017.09.005
Rooksby, H.P. and Lewis, B., J. Less-Common Met., 1964, vol. 6, p. 451. https://doi.org/10.1016/0022-5088(64)90090-6
van Reuth, E.C. and Waterstrat, R.M., Acta Crystallogr., Sect. B, 1968, vol. 24, p. 186. https://doi.org/10.1107/S0567740868001937
Topic, M., Khumalo, Z., and Pineda-Vargas, C.A., Nucl. Instrum. Methods Phys. Res., Sect. B, 2014, vol. 318, p. 163. https://doi.org/10.1016/j.nimb.2013.06.047
Fesik, E.V., Buslaeva, T.M., and Melnikova, T.I., Russ. J. Gen. Chem., 2017, vol. 87, no. 2, p. 159. https://doi.org/10.1134/S1070363217020013
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Fesik, E.V., Buslaeva, T.M., Melnikova, T.I. et al. Solid-Phase Reaction of Tetraammineplatinum(II) Chloride with Ammonium Heptamolybdate. Russ J Gen Chem 90, 1020–1024 (2020). https://doi.org/10.1134/S1070363220060134
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363220060134