Abstract
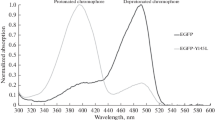
The site-directed mutagenesis of the monomeric red fluorescent protein TagRFP and its variants was performed with the goal of generating reversibly photoactivatable fluorescent proteins. Amino acids at positions 69, 148, 165, 179, and 181 (enumeration according to the green fluorescent protein GFP) were shown to play a key role in the manifestation of the photoactivatable properties. A reversibly photoactivatable red fluorescent protein KFP-HC with excitation and emission maxima at 585 and 615 nm, respectively, was generated. The KFP-HC fluorescent intensity was decreased by 5–10 times under green light (530–560 nm) irradiation (due to the fall of the fluorescence quantum yield) and restored under irradiation with blue light (450–490 nm) or after incubation in the dark (recovery half-time of 30 min).
Similar content being viewed by others
Abbreviations
- GFP:
-
green fluorescent protein
- KFP1:
-
kindling fluorescent protein
- PA-GFP:
-
photoactivatable green fluorescent protein
- PS-CFP:
-
photoswitchable cyan fluorescent protein
- PAFP:
-
photoactivatable fluorescent proteins
References
Pakhomov, A.A. and Martynov, V.I., Chem. Biol., 2008, vol. 15, pp. 755–764.
Chalfie, M., Tu, Y., Euskirchen, G., Ward, W.W., and Prasher, D.C., Science, 1994, vol. 263, pp. 802–805.
Tsien, R.Y., Annu. Rev. Biochem., 1998, vol. 67, pp. 509–544.
Matz, M.V., Fradkov, A.F., Labas, Y.A., Savitsky, A.P., Zaraisky, A.G., Markelov, M.L., and Lukyanov, S.A., Nat. Biotechnol., 1999, vol. 17, pp. 969–973.
Shagin, D.A., Barsova, E.V., Yanushevich, Y.G., Fradkov, A.F., Lukyanov, K.A., Labas, Y.A., Semenova, T.N., Ugalde, J.A., Meyers, A., Nunez, J.M., Widder, E.A., Lukyanov, S.A., and Matz, M.V., Mol. Biol. Evol., 2004, vol. 21, pp. 841–850.
Deheyn, D.D., Kubokawa, K., McCarthy, J.K., Murakami, A., Porrachia, M., Rouse, G.W., and Holland, N.D., Biol. Bull., 2007, vol. 213, pp. 95–100.
Chudakov, D.M., Lukyanov, S., and Lukyanov, K.A., Trends Biotechnol., 2005, vol. 23, pp. 605–613.
Chudakov, D.M. and Luk’yanov, K.A., Biokhimiya, 2003, vol. 68, pp. 1166–1172.
Lukyanov, K.A., Chudakov, D.M., Lukyanov, S., and Verkhusha, V.V., Nat. Rev. Mol. Cell Biol., 2005, vol. 6, pp. 885–891.
Patterson, G.H. and Lippincott-Schwartz, J., Science, 2002, vol. 13, pp. 1873–1877.
Chudakov, D.M., Verkhusha, V.V., Staroverov, D.B., Souslova, E.A., Lukyanov, S., and Lukyanov, K.A., Nat. Biotechnol., 2004, vol. 22, pp. 1435–1439.
van Thor, J.J., Gensch, T., Hellingwerf, K.J., and Johnson, L.N., Nat. Struct. Biol., 2002, vol. 9, pp. 37–41.
Henderson, J.N., Gepshtein, R., Heenan, J.R., Kallio, K., Huppert, D., and Remington, S.J., J. Am. Chem. Soc., 2009, vol. 131, pp. 4176–4177.
Lukyanov, K.A., Fradkov, A.F., Gurskaya, N.G., Matz, M.V., Labas, Y.A., Savitsky, A.P., Markelov, M.L., Zaraisky, A.G., Zhao, X., Fang, Y., Tan, W., and Lukyanov, S.A., J. Biol. Chem., 2000, vol. 275, pp. 25879–25882.
Chudakov, D.M., Belousov, V.V., Zaraisky, A.G., Novoselov, V.V., Staroverov, D.B., Zorov, D.B., Lukyanov, S., and Lukyanov, K.A., Nat. Biotechnol., 2003, vol. 21, pp. 191–194.
Chudakov, D.M., Feofanov, A.V., Mudrik, N.N., Lukyanov, S., and Lukyanov, K.A., J. Biol. Chem., 2003, vol. 278, pp. 7215–7219.
Ando, R., Mizuno, H., and Miyawaki, A., Science, 2004, vol. 306, pp. 1370–1373.
Henderson, J.N., Ai, H.W., Campbell, R.E., and Remington, S.J., Proc. Natl. Acad. Sci. USA, 2007, vol. 104, pp. 6672–6677.
Stiel, A.C., Trowitzsch, S., Weber, G., Andresen, M., Eggeling, C., Hell, S.W., Jakobs, S., and Wahl, M.C., Biochem. J., 2007, vol. 402, pp. 35–42.
Stiel, A.C., Andresen, M., Bock, H., Hilbert, M., Schilde, J., Schonle, A., Eggeling, C., Egner, A., Hell, S.W., and Jakobs, S., Biophys. J., 2008, vol. 95, pp. 2989–2997.
Shaner, N.C., Campbell, R.E., Steinbach, P.A., Giepmans, B.N., Palmer, A.E., and Tsien, R.Y., Nat. Biotechnol., 2004, vol. 22, pp. 1567–1572.
Andresen, M., Wahl, M.C., Stiel, A.C., Grater, F., Schafer, L.V., Trowitzsch, S., Weber, G., Eggeling, C., Grubmuller, H., Hell, S.W., and Jakobs, S., Proc. Natl. Acad. Sci. USA, 2005, vol. 102, pp. 13070–13074.
Andresen, M., Stiel, A.C., Trowitzsch, S., Weber, G., Eggeling, C., Wahl, M.C., Hell, S.W., and Jakobs, S., Proc. Natl. Acad. Sci. USA, 2007, vol. 104, pp. 13005–13009.
Betzig, E., Patterson, G.H., Sougrat, R., Lindwasser, O.W., Olenych, S., Bonifacino, J.S., Davidson, M.W., Lippincott-Schwartz, J., and Hess, H.F., Science, 2006, vol. 313, pp. 1642–1645.
Hofmann, M., Eggeling, C., Jakobs, S., and Hell, S.W., Proc. Natl. Acad. Sci. USA, 2005, vol. 102, pp. 17565–17569.
Schwentker, M.A., Bock, H., Hofmann, M., Jakobs, S., Bewersdorf, J., Eggeling, C., and Hell, S.W., Microsc. Res. Tech., 2007, vol. 70, pp. 269–280.
Egner, A., Geisler, C., von Middendorff, C., Bock, H., Wenzel, D., Medda, R., Andresen, M., Stiel, A.C., Jakobs, S., Eggeling, C., Schönle, A., and Hell, S.W., Biophys. J., 2007, vol. 93, pp. 3285–3290.
Hell, S.W., Science, 2007, vol. 316, pp. 1153–1158.
Merzlyak, E.M., Goedhart, J., Shcherbo, D., Bulina, M.E., Shcheglov, A.S., Fradkov, A.F., Gaintzeva, A., Lukyanov, K.A., Lukyanov, S., Gadella, T.W., and Chudakov, D.M., Nat. Methods, 2007, vol. 4, pp. 555–557.
Ho, S.N., Hunt, H.D., Horton, R.M., Pullen, J.K., and Pease, L.R., Gene, 1989, vol. 77, pp. 51–59.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © L. Zhang, N.G. Gurskaya, Y.E. Kopantseva, N.N. Mudrik, L.L. Vagner, K.A. Lukyanov, D.M. Chudakov, 2010, published in Bioorganicheskaya Khimiya, 2010, Vol. 36, No. 2, pp. 193–199.
Rights and permissions
About this article
Cite this article
Zhang, L., Gurskaya, N.G., Kopantseva, Y.E. et al. Identification of the amino acid residues responsible for the reversible photoconversion of the monomeric red fluorescent protein TagRFP. Russ J Bioorg Chem 36, 179–184 (2010). https://doi.org/10.1134/S1068162010020068
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162010020068