Abstract
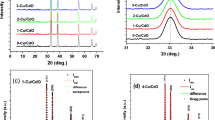
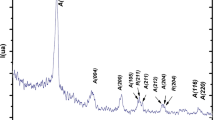
Comparative experimental data, using dynamic light scattering methods, scanning electron and atomic force microscopy, on the composition, crystal structure, semiconductor and photoelectric properties of PbSe films deposited at the presence of sodium sulfite Na2SO3 and “sodium sulfite–ascorbic acid” complex antioxidant additive are presented. Significant changes in morphology, elemental and phase composition, the period of the crystal lattice of PbSe films been synthesized in the presence of Na2SO3 and Na2SO3 + C6H8O6 anti-oxidants before and after their thermoactivation at 653 K are found. It is shown that the introduction of a complex additive in comparison with Na2SO3 increases the homogeneity of the particles forming the layer, decreases the microstresses in it, reduces the value of thermal band gap from 0.349 to 0.308 eV, and increases the volt-watt sensitivity of the films by 28–30%.








Similar content being viewed by others
REFERENCES
V. G. Butkevich, V. D. Bochkov, and E. R. Globus, Prikl. Fiz., No. 6, 66 (2001).
S. P. Zimin and E. S. Gorlachev, Nanostructured Lead Chalcogenides (YarGU, Yaroslavl’, 2011) [in Russian].
D. Scaccabarozzi, B. Saggin, D. Baruffaldi, and M. Tarabini, Measurement 80, 108 (2016).
H. Lee, C. Oh, and J. W. Hahn, Infrared Phys. Technol. 57, 50 (2013).
C. Sierra, M. C. Torquemada, G. Vergara, M. T. Rodrigo, C. Gutiérrez, G. Pérez, I. Génova, I. Catalán, L. J. Gómez, V. Villamayor, M. Álvarez, D. Fernández, M. T. Magaz, and R. M. Almazán, Sens. Actuators, B 190, 464 (2014).
G. E. Rachkovskaya, A. M. Malyarevich, and G. B. Zakharevich, Tr. BGTU, Ser. 3: Khim. Tekhnol. Neorg. V-v. 1, 152 (2010).
S. Anwar, M. Pattanaik, B. K. Mishra, and S. Anwar, Mater. Sci. Semicond. Proc. 34, 45 (2015).
O. E. Semonin, J. M. Luther, and M. C. Beard, Mater. Today 14, 508 (2012).
V. Arivazhagan, M. M. Parvathi, and S. Rajesh, Vacuum 86, 1092 (2012).
X. J. Wang, Y. B. Hou, Y. Chang, C. R. Becker, R. F. Klie, R. Kodama, F. Aqariden, and S. Sivananthan, J. Cryst. Growth 2010, 910 (2010).
X. Sun, K. Gao, X. Pang, H. Yang, and A. A. Volinsky, Thin Solid Films 592, 59 (2015).
N. Ghobadi and E. G. Hatam, J. Cryst. Growth 418, 111 (2015).
V. F. Markov, N. A. Tretyakova, L. N. Maskaeva, V. M. Bakanov, and H. N. Mukhamedzyanov, Thin Solid Films 520, 5227 (2012).
S. Wang, T. Shen, H. Bai, B. Li, and J. Tian, J. Mater. Chem. C 34, 8020 (2016).
N. A. Tret’yakova, V. F. Markov, L. N. Maskaeva, and Kh. N. Mukhamedzyanov, Kondens. Sredy Mezhfaz. Granitsy 7, 189 (2005).
G. Almeida, S. Dogan, G. Bertoni, C. Giannini, R. Gaspari, S. Perissinotto, R. Krahne, S. Ghosh, and L. Manna, J. Am. Chem. Soc. 139, 3005 (2017).
V. M. Yurk, L. N. Maskaeva, V. F. Markov, and V. G. Bamburov, Russ. J. Appl. Chem. 92, 394 (2019).
W. Lv, X. Wang, Q. Qiu, F. Wang, Z. Luo, and W. Weng, J. Alloys Compd. 493, 358 (2010).
D. Kim and H. S. Kim, Mater. Lett. 215, 191 (2018).
Y. Wang, Y. Zhang, F. Wang, D. E. Giblin, J. Hoy, H. W. Rohrs, R. A. Loomis, and W. E. Buhro, Chem. Mater. 26, 2233 (2014).
R. W. Crisp, D. M. Kroupa, A. R. Marshall, E. M. Miller, J. Zhang, and M. C. B. J. M. Luther, Sci. Rep. 5, 9945 (2015).
P. Kumar, M. Pfeffer, C. Berthold, and O. Eibl, J. Alloys Compd. 724, 316 (2017).
H. Yang, L. Chen, X. Li, and J. Zheng, Mater. Lett. 169, 273 (2016).
Kh. N. Mukhamedzyanov, M. P. Mironov, S. I. Yagodin, L. N. Maskaeva, and V. F. Markov, Tsvetn. Met. 12, 57 (2009).
L. N. Maskaeva, V. F. Markov, Z. I. Smirnova, D. A. Belousov, and V. M. Yurk, RF Patent No. 2617350, Byull. Izobret., No. 12 (2017).
V. M. Bakanov, Z. I. Smirnova, Kh. N. Mukhamedzyanov, L. N. Maskaeva, and V. F. Markov, Kondens. Sredy Mezhfaz. Granitsy 13, 401 (2011).
Z. I. Smirnova, V. M. Bakanov, L. N. Maskaeva, V. F. Markov, and V. I. Voronin, Phys. Solid State 56, 2561 (2014).
V. S. Urusov, Isomorphic Miscibility Theory (Nauka, Moscow, 1977) [in Russian].
K. C. Preetha and T. L. Remadevi, Mater. Sci. Semicond. Proc. 16, 605 (2013).
S. A. Bashkirov, V. F. Gremenoka, V. A. Ivanov, K. Bente, P. P. Gladyshev, T. Yu. Zelenyak, A. M. Saad, and M. S. Tivano, Thin Solid Films 616, 773 (2016).
W. Feng, J. Song, Y. Ren, L. Yi, J. Hu, R. Zhu, and H. Dong, Phys. E (Amsterdam, Neth.) 102, 153 (2018).
V. P. Zlomanov, O. I. Tananaeva, and A. V. Novoselova, Zh. Neorg. Khim. 6, 2753 (1961).
A. S. Pashinkin and M. M. Spivak, Neorg. Mater. 24, 133 (1988).
V. V. Tomaev and Yu. V. Petrov, Glass Phys. Chem. 38, 240 (2012).
N. P. Anisimova, N. E. Tropina, and A. N. Tropin, Semiconductors 44, 1554 (2010).
M. F. Panov and V. V. Tomaev, Glass Phys. Chem. 38, 419 (2012).
Kh. N. Mukhamedzyanov, V. F. Markov, and L. N. Maskaeva, Semiconductors 48, 263 (2014).
X. Sun, K. Gao, X. Pang, H. Yang, and A. A. Volinsky, Appl. Surf. Sci. 356, 978 (2015).
H. Yang, X. Li, T. Mei, and J. Zheng, Mater. Lett. 194, 142 (2017).
M. C. Torquemada, M. T. Rodrigo, G. Vergara, F. J. Sranchez, R. Almazran, M. Verdru, P. Rodrrıguez, V. Villamayor, L. J. Gromez, and M. T. Montojo, J. Appl. Phys. 93, 1778 (2003).
H. Yang, X. Li, G. Wang, and J. Zheng, AIP Adv. 8, 085316 (2018).
L. Zhao, J. Qiu, B. Weng, C. Chang, Z. Yuan, and Z. Shi, J. Appl. Phys. 115, 084502 (2014).
Ch. E. Ekuma, D. J. Singh, J. Moreno, and M. Jarrell, Phys. Rev. B 85, 085205 (2012).
D. V. Shtanskii, S. A. Kulinich, E. A. Levashov, and J. J. Moore, Phys. Solid State 45, 1177 (2003).
Yu. I. Golovin, Phys. Solid State 50, 2205 (2008).
E. A. Levashova and D. V. Shtansky, Russ. Chem. Rev. 76, 463 (2007).
M. Park, H. Kim, and J. P. Youngblood, Nanotechnology 19, 055705 (2008).
R. Rahman and P. Servati, Nanotechnology 23, 055703-1 (2012).
N. Titus, Sensors 18, 9 (2001).
Funding
The work was carried out with the financial support of the program 211 of the Government of the Russian Federation no. 02.A03.21.0006, grant 20-48-660041 r_а of the Russian Foundation for Basic Research, and the state assignment of Federal Agency for Scientific Organizations (FANO) of Russia (project “Potok” no. AAAA-A18-118020190112-8).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by S. Rostovtseva
Rights and permissions
About this article
Cite this article
Maskaeva, L.N., Yurk, V.M., Markov, V.F. et al. Effect of “Sodium Sulfite–Ascorbic Acid” Complex Antioxidant Additive on the Composition, Structure and Semiconducting Properties of PbSe Film. Phys. Solid State 62, 1949–1959 (2020). https://doi.org/10.1134/S1063783420100212
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063783420100212