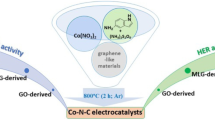
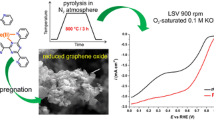
Abstract
It is shown that the use of aqueous electrolytes based on salts of various aromatic carboxylic acids in the course of electrochemical exfoliation of graphite allows producing multilayered graphene (MLG) and protects it from the negative oxidative action of oxygen-containing radicals, in particular, ∙OH radicals formed at water electrolysis on the graphite anode. It is shown that the electrochemically synthesized MLG can be used for the formation of noble-metal-free carbonized nanocomposite Co–N–C electrocatalysts of the oxygen reduction reaction (ORR) and the hydrogen evolution reaction (HER). Such poly-2,6-diaminopyridine-based catalysts in aqueous 0.5 М H2SO4 are shown to surpass their acetylene black-based analogues as regards their functional characteristics in ORR and HER and also are highly tolerant with respect to CO and methanol in the course of oxygen reduction. Based on experimental data, it is concluded that the predominant active centers in Co–N–C electrocatalysts based on MLG and acetylene black are of the different nature and that the same active centers are involved in ORR and HER.









Similar content being viewed by others
REFERENCES
Zhong, Y.L., Tian, Z., Simon, G.P., and Li, D., Scalable production of graphene via wet chemistry: progress and challenges, Mater. Today, 2015, vol. 18, no. 2, p. 73.
Paredes, J.I. and Munuera, J.M., Recent advances and energy-related applications of high quality/chemically doped graphenes obtained by electrochemical exfoliation methods, J. Mater. Chem. A, 2017, vol. 5, p. 7228.
Ambrosi, A. and Pumera, M., Exfoliation of layered materials using electrochemistry, Chem. Soc. Rev., 2018, vol. 47, no. 19, p. 7213.
Parvez, K., Li, R., Puniredd, S.R., Hernandez, Y., Hinkel, F., Wang, S., Feng, X., and Müllen, K., Electrochemically exfoliated graphene as solution-processable, highly conductive electrodes for organic electronics, ACS Nano, 2013, vol. 7, no. 4, p. 3598.
Liu, J., Poh, C.K., Zhan, D., Lai, L., Lim, S.H., Wang, L., Liu, X., Sahoo, N.G., Li, C., Shen, Z., and Lin, J., Improved synthesis of graphene flakes from the multiple electrochemical exfoliation of graphite rod, Nano Energy, 2013, vol. 2, no. 3, p. 377.
Qi, B., He, L., Bo, X., Yang H., and Guo, L., Electrochemical preparation of free-standing few-layer graphene through oxidation–reduction cycling, Chem. Eng. J., 2011, vol. 171, no. 1, p. 340.
Rao, K.S., Senthilnathan, J., Liu, Y.-F., and Yoshimura, M., Role of peroxide ions in formation of graphene nanosheets by electrochemical exfoliation of graphite, Sci. Rep., 2014, vol. 4, p. 4237.
Parvez, K., Wu, Z.-S., Li, R., Liu, X., Graf, R., Feng, X., and Müllen, K., Exfoliation of graphite into graphene in aqueous solutions of inorganic salts, J. Amer. Chem. Soc., 2014, vol. 136, no. 16, p. 6083.
Krivenko, A.G., Manzhos, R.A., Komarova, N.S., Kotkin, A.S., Kabachkov, E.N., and Shul’ga, Yu.M., Comparative study of graphite and the products of its electrochemical exfoliation, Russ. J. Electrochem., 2018, vol. 54, p. 825.
Mahanandia, P., Simon, F., Heinrich, G., and Nanda, K.K., An electrochemical method for the synthesis of few layer graphene sheets for high temperature applications, Chem. Commun., 2014, vol. 50, no. 35, p. 4613.
Ustavytska, O., Kurys, Ya., Koshechko, V., and Pokho-denko, V., One-step electrochemical preparation of multilayer graphene functionalized with nitrogen, Nanoscale Res. Lett., 2017, vol. 12, p. 175.
Zhou, N., Wang, N., Wu, Z., and Li, L., Probing active sites on metal-free, nitrogen-doped carbons for oxygen electroreduction: a review, Catalysts, 2018, vol. 8, p. 509.
Yan, X., Jia, Y., and Yao, X., Defects on carbons for electrocatalytic oxygen reduction, Chem. Soc. Rev., 2018, vol. 47, p. 7628.
Tarasevich, M.R. and Davydova, E.S., Nonplatinum cathodic catalysts for fuel cells with alkaline electrolyte (Review), Russ. J. Electrochem., 2016, vol. 52, p. 193.
Kurys, Ya.I., Ustavytska, O.O., Koshechko, V.G., and Pokhodenko, V.D., Structure and electrochemical properties of multilayer graphene prepared by electrochemical exfoliation of graphite in presence of benzoate ions, RSC Adv., 2016, vol. 6, no. 42, p. 36050.
Organic Electrochemistry: an Introduction and a Guide, 2nd Ed., Baizer, M.M. and Lund, H., Eds., New York: Marcel Dekker, 1983.
Anodic Oxidation. Organic Chemistry: A Series of Monographs, vol. 32, Ross, S.D., Finkelstein, M., and Rudd, E.J., Eds., New York: Academic Press, 1975.
Linstead, R.P., Shephard, B.R., and Weedon, B.C.L., Anodic syntheses. Part VII. Electrolyses of mono-, di-, and tri-phenylacetic acids in non-aqueous solutions, J. Chem. Soc., 1952, p. 3624.
Sandhwar, V.K. and Prasad, B., A comparative study of electrochemical degradation of benzoic acid and terephthalic acid from aqueous solution of purified terephthalic acid (PTA) wastewater, J. Water Process Eng., 2019, vol. 30, p. 100381.
Zhu, Y.P., Guo, C., Zheng, Y., and Qiao, S.-Z., Surface and interface engineering of noble-metal-free electrocatalysts for efficient energy conversion processes, Acc. Chem. Res., 2017, vol. 50, no. 4, p. 915.
Liu, K., Zhong, H., Meng, F., Zhang, X., Yan, J., and Jiang, Q., Recent advances in metal–nitrogen–carbon catalysts for electrochemical water splitting, Mater. Chem. Front., 2017, vol. 1, no. 11, p. 2155.
Gewirth, A.A., Varnell, J.A., and DiAscro, A.M., Nonprecious metal catalysts for oxygen reduction in heterogeneous aqueous systems, Chem. Rev., 2018, vol. 118, no. 5, p. 2313.
He, Y., Tan, Q., Lu, L., Sokolowski, J., and Wu, G., Metal–Nitrogen–Carbon catalysts for oxygen reduction in PEM fuel cells: self-template synthesis approach to enhancing catalytic activity and stability, Electrochem. Energy Rev., 2019, vol. 2, no. 2, p. 231.
Kovtyukhova, N.I., Ollivier, P.J., Martin, B.R., Mallouk, T.E., Chizhik, S.A., Buzaneva, E.V., and Gorchinskiy, A.D., Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations, Chem. Mater., 1999, vol. 11, no. 3, p. 771.
Pariiska, O.O., Mazur, D.O., Kurys, Ya.I., Koshe-chko, V.G., and Pokhodenko, V.D., Effect of the formation conditions on the activity of Co–N–C electrocatalysts derived from poly-m-phenylenediamine in the reduction of oxygen, Theor. Exp. Chem., 2019, vol. 54, no. 6, p. 386.
Rosenau, C.P., Jelier, B.J., Gossert, A.D., and Togni, A., Exposing the origins of irreproducibility in fluorine NMR spectroscopy, Angew. Chem. Int. Ed., 2018, vol. 57, p. 1.
Munuera, J.M., Paredes, J.I., Villar-Rodil, S., Ayán-Varela, M., Martínez-Alonso, A., and Tascón, J.M.D., Electrolytic exfoliation of graphite in water with multifunctional electrolytes: en route towards high quality, oxide-free graphene flakes, Nanoscale, 2016, vol. 8, p. 2982.
Lu, J., Yang, J., Wang, J., Lim, A., Wang, S., and Loh, K.P., One-pot synthesis of fluorescent carbon nanoribbons, nanoparticles, and graphene by the exfoliation of graphite in ionic liquids, ACS Nano, 2009, vol. 3, no. 8, p. 2367.
Das, A., Chakraboty, B., and Sood, A.K., Raman spectroscopy of graphene on different substrates and influence of defects, Bull. Mater. Sci., 2008, vol. 31, no. 3, p. 579.
Min, Y., Shen, Z., Zhang, X., and Ma, S., Achieving concentrated graphene dispersions in water/acetone mixtures by the strategy of tailoring Hansen solubility parameters, J. Phys. D: Appl. Phys., 2013, vol. 46, no. 2, p. 025301.
López, V., Sundaram, R.S., Gómez-Navarro, C., Olea, D., Burghard, M., Gómez-Herrero, J., Zamora, F., and Kern, K., Chemical vapor deposition repair of graphene oxide: a route to highly-conductive graphene monolayers, Adv. Mater., 2009, vol. 21, no. 46, p. 4683.
Mattevi, C., Eda, G., Agnoli, S., Miller, S., Mkhoyan, K.A., Celik, O., Mastrogiovanni, D., Granozzi, G., Garfunkel, E., and Chhowalla, M., Evolution of electrical, chemical, and structural properties of transparent and conducting chemically derived graphene thin films, Adv. Funct. Mater., 2009, vol. 19, no. 16, p. 2577.
Wang, S., Dou, S., Tao, L., Huo, J., and Dai, L., Etched and doped Co9S8/graphene hybrid for oxygen electrocatalysis, Energy Environ. Sci., 2016, vol. 9, p. 1320.
Zhang, L., Liu, W., Dou, Y., Du., Z., and Shao, M., The role of transition metal and nitrogen in Metal–N–C composites for hydrogen evolution reaction at universal pHs, J. Phys. Chem. C, 2016, vol. 120, no. 51, p. 29047.
Wang, X., Ke, Y., Pan, H., Ma, K., Xiao, Q., Yin, D., Wu, G., and Swihart, M.T., Cu-deficient plasmonic Cu2–xS nanoplate electrocatalysts for oxygen reduction, ACS Catal., 2015, vol. 5, no. 4, p. 2534.
Zhang, Q., Mamtani, K., Jain, D., Ozkan, U., and Asthagiri, A., CO poisoning effects on FeNC and CNx ORR catalysts: A combined experimental–computational study, J. Phys. Chem. C, 2016, vol. 120, no. 28, p. 15173.
Zhang, J., Chen, G., Müllen, K., and Feng, X., Carbon-rich nanomaterials: Fascinating hydrogen and oxygen electrocatalysts, Adv. Mater., 2018, vol. 30, 1800528.
Shinagawa, T., Garcia-Esparza, A.T., and Takanabe, K., Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion, Sci. Rep., 2015, vol. 5, 13801.
Funding
This study is partly supported by the targeted complex programs of scientific research of the National Academy of Sciences (NAN) of Ukraine “Fundamental Aspects of Renewable Hydrogen Energetics and Fuel Cell Technologies” and “New Functional Substances and Materials for Chemical Industry” and also by the targeted complex program of fundamental research of the NAN of Ukraine “Fundamental Problems of the Development of New Nanomaterials and Nanotechnologies.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare the absence of conflict of interests.
Additional information
Translated by T. Safonova
Published on the basis of materials of the XIX All-Russian Conference “Electrochemistry of Organic Compounds” (EKHOS-2018) (with international participation), Novocherkassk, 2018.
Rights and permissions
About this article
Cite this article
Kurys, Y.I., Pariiska, O.O., Mazur, D.O. et al. Electrochemical Synthesis of Multilayered Graphene and Its Use in Co–N–C Electrocatalysts of Oxygen Reduction and Hydrogen Evolution. Russ J Electrochem 56, 271–284 (2020). https://doi.org/10.1134/S1023193520040072
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193520040072