Out of clutter, find simplicity.
From discord, find harmony.
In the middle of difficulty lies opportunity.
Albert Einstein
Abstract
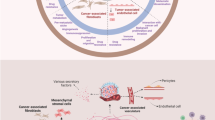
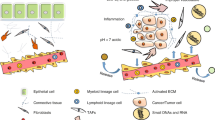
The review attempts to discuss the interaction of cancer cells and cells of the tumor microenvironment as well as the possibility of using them as a target for antitumor therapy.



Similar content being viewed by others
REFERENCES
Sverdlov, E.D., Genetic surgery—a right strategy to attack cancer, Curr. Gene Ther., 2011, vol. 11, no. 6, pp. 501—531.
Sverdlov, E.D., Multidimensional complexity of cancer: simple solutions are needed, Biochemistry (Moscow), 2016, vol. 81, no. 7, pp. 731—738. https://doi.org/.10.1134/S0006297916070099.
Alizadeh, A.A., Aranda, V., Bardelli, A., et al., Toward understanding and exploiting tumor heterogeneity, Nat. Med., 2015, vol. 21, no. 8, pp. 846—853.
Schnekenburger, M., Florean, C., Dicato, M., and Diederich, M., Epigenetic alterations as a universal feature of cancer hallmarks and a promising target for personalized treatments, Curr. Top Med. Chem., 2016, vol. 16, no. 7, pp. 745—776. doi 10.2174/1568026615666150825141330
Hanahan, D. and Weinberg, R.A., The hallmarks of cancer, Cell, 2000, vol. 100, no. 1, pp. 57—70.
Ramamonjisoa, N. and Ackerstaff, E., Characterization of the tumor microenvironment and tumor—stroma interaction by non-invasive preclinical imaging, Front. Oncol., 2017, vol. 7, p. 3. doi 10.3389/fonc.2017.00003
Weinberg, R.A., Coming full circle—from endless complexity to simplicity and back again, Cell, 2014, vol. 157, no. 1, pp. 267—271. doi 10.1016/j.cell.2014.03.004
Hanahan, D. and Coussens, L.M., Accessories to the crime: functions of cells recruited to the tumor microenvironment, Cancer Cell, 2012, vol. 21, no. 3, pp. 309—322. doi 10.1016/j.ccr.2012.02.022
Hanahan, D. and Weinberg, R.A., Hallmarks of cancer: the next generation, Cell, 2011, vol. 144, no. 5, pp. 646—674.
Horne, S.D., Pollick, S.A., and Heng, H.H., Evolutionary mechanism unifies the hallmarks of cancer, Int. J. Cancer, 2015, vol. 136, no. 9, pp. 2012—2021. doi 10.1002/ijc.29031
Fouad, Y.A. and Aanei, C., Revisiting the hallmarks of cancer, Am. J. Cancer Res., 2017, vol. 7, no. 5, pp. 1016—1036.
Orimo, A. and Weinberg, R.A., Stromal fibroblasts in cancer: a novel tumor-promoting cell type, Cell Cycle, 2006, vol. 5, no. 15, pp. 1597—1601. doi 10.4161/cc.5.15.3112
Gonda, T.A., Varro, A., Wang, T.C., and Tycko, B., Molecular biology of cancer-associated fibroblasts: can these cells be targeted in anti-cancer therapy?, Semin. Cell Dev. Biol., 2010, vol. 21, no. 1, pp. 2—10. doi 10.1016/j.semcdb.2009.10.001
De Palma, M. and Hanahan, D., The biology of personalized cancer medicine: facing individual complexities underlying hallmark capabilities, Mol. Oncol., 2012, vol. 6, no. 2, pp. 111—127. doi 10.1016/j.molonc.2012.01.011
Gascard, P. and Tlsty, T.D., Carcinoma-associated fibroblasts: orchestrating the composition of malignancy, Genes Dev., 2016, vol. 30, no. 9, pp. 1002—1019. doi 10.1101/gad.279737.116
Chen, F., Zhuang, X., Lin, L., et al., New horizons in tumor microenvironment biology: challenges and opportunities, BMC Med., 2015, vol. 13, p. 45.
Gandellini, P., Andriani, F., Merlino, G., et al., Complexity in the tumour microenvironment: cancer associated fibroblast gene expression patterns identify both common and unique features of tumour—stroma crosstalk across cancer types, Semin. Cancer Biol., 2015, vol. 35, pp. 96—106. doi 10.1016/j.semcancer.2015.08.008
Stadler, M., Walter, S., Walzl, A., et al., Increased complexity in carcinomas: Analyzing and modeling the interaction of human cancer cells with their microenvironment, Semin. Cancer Biol., 2015, vol. 35, pp. 107—124. doi 10.1016/j.semcancer.2015.08.007
Zi, F., He, J., He, D., et al., Fibroblast activation protein alpha in tumor microenvironment: recent progression and implications (review), Mol. Med. Rep., 2015, vol. 11, no. 5, pp. 3203—3211.
Raffaghello, L. and Dazzi, F., Classification and biology of tumour associated stromal cells, Immunol. Lett., 2015, vol. 168, no. 2, pp. 175—182. doi 10.1016/j.imlet.2015.06.016
Bizzarri, M. and Cucina, A., Tumor and the microenvironment: a chance to reframe the paradigm of carcinogenesis?, Biomed. Res. Int., 2014, vol. 2014, p. 934038. doi 10.1155/2014/934038
Reina-Campos, M., Moscat, J., and Diaz-Meco, M., Metabolism shapes the tumor microenvironment, Curr. Opin. Cell Biol., 2017, vol. 48, pp. 47—53. doi 10.1016/j.ceb.2017.05.006
Sadelain, M., Riviere, I., and Brentjens, R., Targeting tumours with genetically enhanced t lymphocytes, Nat. Rev. Cancer, 2003, vol. 3, no. 1, pp. 35—45. doi 10.1038/nrc971
Schumacher, T.N. and Schreiber, R.D., Neoantigens in cancer immunotherapy, Science, 2015, vol. 348, no. 6230, pp. 69—74.
Martin, S.D., Coukos, G., Holt, R.A., and Nelson, B.H., Targeting the undruggable: immunotherapy meets personalized oncology in the genomic era, Ann. Oncol., 2015, vol. 26, no. 12, pp. 2367—2374.
Ziani, L., Chouaib, S., and Thiery, J., Alteration of the antitumor immune response by cancer-associated fibroblasts, Front. Immunol., 2018, vol. 9, p. 414. doi 10.3389/fimmu.2018.00414
van der Burg, S.H., Arens, R., Ossendorp, F., et al., Vaccines for established cancer: overcoming the challenges posed by immune evasion, Nat. Rev. Cancer, 2016, vol. 16, no. 4, pp. 219—233.
Muenst, S., Laubli, H., Soysal, S.D., et al., The immune system and cancer evasion strategies: therapeutic concepts, J. Int. Med., 2016, vol. 279, no. 6, pp. 541—562.
Schreiber, R.D., Old, L.J., and Smyth, M.J., Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion, Science, 2011, vol. 331, no. 6024, pp. 1565—1570.
Escors, D., Tumour immunogenicity, antigen presentation and immunological barriers in cancer immunotherapy, New J. Sci., 2014, vol. 2014. doi 10.1155/2014/734515
Stambrook, P.J., Maher, J., and Farzaneh, F., Cancer immunotherapy: whence and whither, Mol. Cancer Res., 2017, vol. 15, no. 6, pp. 635—650. doi 10.1158/1541-7786.MCR-16-0427
Baumeister, S.H., Freeman, G.J., Dranoff, G., and Sharpe, A.H., Coinhibitory pathways in immunotherapy for cancer, Annu. Rev. Immunol., 2016, vol. 34, pp. 539—573. doi 10.1146/annurev-immunol-032414-112049
Marin-Acevedo, J.A., Dholaria, B., Soyano, A.E., et al., Next generation of immune checkpoint therapy in cancer: New developments and challenges, J. Hematol. Oncol., 2018, vol. 11, no. 1, p. 39. doi 10.1186/s13045-018-0582-8
Marcucci, F., Rumio, C., and Corti, A., Tumor cell-associated immune checkpoint molecules—drivers of malignancy and stemness, Biochim. Biophys. Acta, 2017, vol. 1868, no. 2, pp. 571—583. doi 10.1016/j.bbcan.2017.10.006
Sharma, P. and Allison, J.P., The future of immune checkpoint therapy, Science, 2015, vol. 348, no. 6230, pp. 56—61.
Sharpe, A.H., Introduction to checkpoint inhibitors and cancer immunotherapy, Immunol. Rev., 2017, vol. 276, no. 1, pp. 5—8. doi 10.1111/imr.12531
Allison, J.P., Immune checkpoint blockade in cancer therapy: the 2015 Lasker—Debakey clinical medical research award, JAMA, 2015, vol. 314, no. 11, pp. 1113—1114. doi 10.1001/jama.2015.11929
Villaruz, L.C., Kalyan, A., Zarour, H., and Socinski, M.A., Immunotherapy in lung cancer, Transl. Lung Cancer Res., 2014, vol. 3, no. 1, pp. 2—14. doi 10.3978/j.issn.2218-6751.2013.10.13
Munhoz, R.R. and Postow, M.A., Clinical development of pd-1 in advanced melanoma, Cancer J., 2018, vol. 24, no. 1, pp. 7—14. doi 10.1097/PPO.0000000000000299
Postow, M. and Wolchok, J., Toxicities associated with checkpoint inhibitor immunotherapy, 2016. http://www.uptodate.com/contents/toxicities-associated-withcheckpoint-inhibitor-immunotherapy. Accessed February 22, 2018.
Alexander, W., The checkpoint immunotherapy revolution: what started as a trickle has become a flood, despite some daunting adverse effects; new drugs, indications, and combinations continue to emerge, P. T., 2016, vol. 41, no. 3, pp. 185—191.
Calabrese, L. and Velcheti, V., Checkpoint immunotherapy: good for cancer therapy, bad for rheumatic diseases, Ann. Rheum. Dis., 2017, vol. 76, no. 1, pp. 1—3. doi 10.1136/annrheumdis-2016-209782
Madden, D.L., From a patient advocate’s perspective: does cancer immunotherapy represent a paradigm shift?, Curr. Oncol. Rep., 2018, vol. 20, no. 1, p. 8. doi 10.1007/s11912-018-0662-5
Abdin, S.M., Zaher, D.M., Arafa, E.A., and Omar, H.A., Tackling cancer resistance by immunotherapy: updated clinical impact and safety of pd-1/pd-l1 inhibitors, Cancers (Basel), 2018, vol. 10, no. 2. doi 10.3390/cancers10020032
Postow, M.A. and Hellmann, M.D., Adverse events associated with immune checkpoint blockade, N. Engl. J. Med., 2018, vol. 378, no. 12, p. 1165.
Brown, J.S., Sundar, R., and Lopez, J., Combining DNA damaging therapeutics with immunotherapy: more haste, less speed, Br. J. Cancer., 2018, vol. 118, no. 3, pp. 312—324. doi 10.1038/bjc.2017.376
Hosoi, A., Takeda, K., Nagaoka, K., et al., Increased diversity with reduced “Diversity evenness” of tumor infiltrating t-cells for the successful cancer immunotherapy, Sci. Rep., 2018, vol. 8, no. 1, p. 1058. doi 10.1038/s41598-018-19548-y
Perrimon, N., Pitsouli, C., and Shilo, B.Z., Signaling mechanisms controlling cell fate and embryonic patterning, Cold Spring Harbor Perspect. Biol., 2012, vol. 4, no. 8. a005975. doi 10.1101/cshperspect.a005975
Sundar, R., Chenard-Poirier, M., Collins, D.C., and Yap, T.A., Imprecision in the era of precision medicine in non-small cell lung cancer, Front. Med. (Lausanne), 2017, vol. 4, p. 39. doi 10.3389/fmed.2017.00039
Minocha, S. and Das, S., Gleevec (imatinib): a breakthrough in cancer treatment, J. Drug Discovery Ther., 2017, vol. 5, no. 8, pp. 13—17.
Mallick, P., Complexity and information: cancer as a multi-scale complex adaptive system, in Physical Science and Engineering Advances in Life Science and Oncology, 2015, pp. 5—29.
Prasad, V., Perspective: the precision-oncology illusion, Nature, 2016, vol. 537, no. 7619, p. S63. doi 10.1038/537S63a
Tannock, I.F. and Hickman, J.A., Limits to personalized cancer medicine, N. Engl. J. Med., 2016, vol. 375, no. 13, pp. 1289—1294. doi 10.1056/NEJMsb1607705
Haynes, B., Sarma, A., Nangia-Makker, P., and Shekhar, M.P., Breast cancer complexity: implications of intratumoral heterogeneity in clinical management, Cancer Metastasis Rev., 2017, vol. 36, no. 3, pp. 547—555. doi 10.1007/s10555-017-9684-y
Taube, J.M., Anders, R.A., Young, G.D., et al., Colocalization of inflammatory response with b7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape, Sci. Transl. Med., 2012, vol. 4, no. 127, pp. 127—137. doi 10.1126/scitranslmed.3003689
Cogdill, A.P., Andrews, M.C., and Wargo, J.A., Hallmarks of response to immune checkpoint blockade, Br. J. Cancer, 2017, vol. 117, no. 1, pp. 1—7. doi 10.1038/bjc.2017.136
Teng, M.W., Ngiow, S.F., Ribas, A., and Smyth, M.J., Classifying cancers based on t-cell infiltration and pd-l1, Cancer Res., 2015, vol. 75, no. 11, pp. 2139—2145. doi 10.1158/0008-5472.CAN-15-0255
Zhang, Y. and Chen, L., Classification of advanced human cancers based on tumor immunity in the microenvironment (time) for cancer immunotherapy, JAMA Oncol., 2016, vol. 2, no. 11, pp. 1403—1404. doi 10.1001/jamaoncol.2016.2450
Koh, J., Ock, C.Y., Kim, J.W., et al., Clinicopathologic implications of immune classification by pd-l1 expression and cd8-positive tumor-infiltrating lymphocytes in stage ii and iii gastric cancer patients, Oncotarget, 2017, vol. 8, no. 16, pp. 26356—26367. doi 10.18632/oncotarget.15465
Ock, C.Y., Keam, B., Kim, S., et al., Pan-cancer immunogenomic perspective on the tumor microenvironment based on pd-l1 and cd8 t-cell infiltration, Clin. Cancer Res., 2016, vol. 22, no. 9, pp. 2261—2270. doi 10.1158/1078-0432.CCR-15-2834
Loo, K. and Daud, A., Emerging biomarkers as predictors to anti-pd1/pd-l1 therapies in advanced melanoma, Immunotherapy, 2016, vol. 8, no. 7, pp. 775—784. doi 10.2217/imt-2016-0039
Dempke, W.C.M., Fenchel, K., Uciechowski, P., and Dale, S.P., Second- and third-generation drugs for immuno-oncology treatment—the more the better?, Eur. J. Cancer, 2017, vol. 74, pp. 55—72. doi 10.1016/j.ejca.2017.01.001
Fearon, D.T., The carcinoma-associated fibroblast expressing fibroblast activation protein and escape from immune surveillance, Cancer Immunol. Res., 2014, vol. 2, no. 3, pp. 187—193. doi 10.1158/2326-6066.CIR-14-0002
Day, D., Monjazeb, A.M., Sharon, E., et al., From famine to feast: developing early-phase combination immunotherapy trials wisely, Clin. Cancer Res., 2017, vol. 23, no. 17, pp. 4980—4991. doi 10.1158/1078-0432.CCR-16-3064
Lamprecht, S., Sigal-Batikoff, I., Shany, S., et al., Teaming up for trouble: cancer cells, transforming growth factor-beta1 signaling and the epigenetic corruption of stromal naive fibroblasts, Cancers (Basel), 2018, vol. 10, no. 3. doi 10.3390/cancers10030061
Kalluri, R., The biology and function of fibroblasts in cancer, Nat. Rev. Cancer, 2016, vol. 16, no. 9, pp. 582—598. doi 10.1038/nrc.2016.73
Shiga, K., Hara, M., Nagasaki, T., et al., Cancer-associated fibroblasts: their characteristics and their roles in tumor growth, Cancers (Basel), 2015, vol. 7, no. 4, pp. 2443—2458. doi 10.3390/cancers7040902
Pidsley, R., Zotenko, E., Peters, T.J., et al., Critical evaluation of the illumina methylationepic beadchip microarray for whole-genome DNA methylation profiling, Genome Biol., 2016, vol. 17, no. 1, p. 208. doi 10.1186/s13059-016-1066-1
Liao, Z., Tan, Z.W., Zhu, P., and Tan, N.S., Cancer-associated fibroblasts in tumor microenvironment— accomplices in tumor malignancy, Cell. Immunol., 2018. doi 10.1016/j.cellimm.2017.12.003
Tran, E., Chinnasamy, D., Yu, Z., et al., Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia, J. Exp. Med., 2013, vol. 210, no. 6, pp. 1125—1135. doi 10.1084/jem.20130110
Bhome, R., Al Saihati, H.A., Goh, R.W., et al., Translational aspects in targeting the stromal tumour microenvironment: from bench to bedside, New Horiz. Transl. Med., 2016, vol. 3, no. 1, pp. 9—21. doi 10.1016/j.nhtm.2016.03.001
Kumar, V., Patel, S., Tcyganov, E., and Gabrilovich, D.I., The nature of myeloid-derived suppressor cells in the tumor microenvironment, Trends Immunol., 2016, vol. 37, no. 3, pp. 208—220. doi 10.1016/j.it.2016.01.004
Jacobs, J., Smits, E., Lardon, F., et al., Immune checkpoint modulation in colorectal cancer: what’s new and what to expect, J. Immunol. Res., 2015, vol. 2015, p. 158038. doi 10.1155/2015/158038
Lakins, M.A., Ghorani, E., Munir, H., et al., Cancer-associated fibroblasts induce antigen-specific deletion of cd8 (+) t cells to protect tumour cells, Nat. Commun., 2018, vol. 9, no. 1, p. 948. doi 10.1038/s41467-018-03347-0
Rozali, E.N., Hato, S.V., Robinson, B.W., et al., Programmed death ligand 2 in cancer-induced immune suppression, Clin. Dev. Immunol., 2012, vol. 2012, p. 656340. doi 10.1155/2012/656340
Nazareth, M.R., Broderick, L., Simpson-Abelson, M.R., et al., Characterization of human lung tumor-associated fibroblasts and their ability to modulate the activation of tumor-associated t cells, J. Immunol., 2007, vol. 178, no. 9, pp. 5552—5562.
Khalili, J.S., Liu, S., Rodriguez-Cruz, T.G., et al., Oncogenic braf(v600e) promotes stromal cell-mediated immunosuppression via induction of interleukin-1 in melanoma, Clin. Cancer Res., 2012, vol. 18, no. 19, pp. 5329—5340. doi 10.1158/1078-0432.CCR-12-1632
Costa, A., Kieffer, Y., Scholer-Dahirel, A., et al., Fibroblast heterogeneity and immunosuppressive environment in human breast cancer, Cancer Cell, 2018, vol. 33, no. 3, pp. 463—479. e410. doi 10.1016/j.ccell.2018. 01.011
Choe, C., Shin, Y.S., Kim, S.H., et al., Tumor-stromal interactions with direct cell contacts enhance motility of non-small cell lung cancer cells through the hedgehog signaling pathway, Anticancer Res., 2013, vol. 33, no. 9, pp. 3715—3723.
Yamaguchi, H. and Sakai, R., Direct interaction between carcinoma cells and cancer associated fibroblasts for the regulation of cancer invasion, Cancers (Basel), 2015, vol. 7, no. 4, pp. 2054—2062. doi 10.3390/cancers7040876
Semba, S., Kodama, Y., Ohnuma, K., et al., Direct cancer-stromal interaction increases fibroblast proliferation and enhances invasive properties of scirrhous-type gastric carcinoma cells, Br. J. Cancer, 2009, vol. 101, no. 8, pp. 1365—1373. doi 10.1038/sj.bjc. 6605309
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by M. Novikova
Rights and permissions
About this article
Cite this article
Alekseenko, I.V., Monastyrskaya, G.S. & Sverdlov, E.D. Emerging Potential of Cancer Therapy—Binary Direct Interactions of Cancer and Stromal Cells. Russ J Genet 54, 1416–1428 (2018). https://doi.org/10.1134/S1022795418120025
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795418120025