Abstract
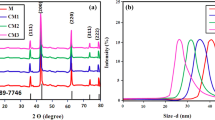
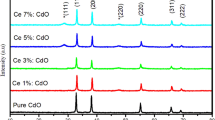
In this study, the characterization and photocatalytic activity of MoO3 nanoparticles doped with various doping concentrations of cerium have been investigated. The Fourier transform infrared (FT-IR) spectra of the prepared catalysts confirmed that MoO3 particles have been successfully doped by cerium. Field emission scanning electron microscopy (FESEM) was performed to visualize the surface morphology of the obtained catalysts. The XRD patterns suggested that the crystallinity of the sample with the lowest doping concentration of 15 mol % was higher in comparison with samples of higher doping concentrations. The volume-averaged crystal sizes of the obtained catalysts were calculated to be 25, 28, and 32 nm for 15, 35, and 60 mol % samples, respectively. The photocatalytic activity along with the reaction kinetics of Ce-doped MoO3 nanoparticles have also been investigated through the dye degradation of methyl orange. The synthesized Ce-doped MoO3 particles with the lowest dopant concentration of 15 mol % exhibited the highest photocatalytic activity for methyl orange dye degradation. It was observed that photo-degradation activity decreased with an increase in the doping concentration of cerium. The predicted rate constants for samples with 15, 35, and 60 mol % doping concentrations were found to be 0.0432, 0.035, and 0.029 min–1, respectively.
Similar content being viewed by others
References
Z. Li, J. Wilcoxon, F. Yin, Y. Chen, R. Palmer, and R. Johnston, Faraday Discuss. 138, 363 (2008)
V. R. Stamenkovic, B. Fowler, B. S. Mun, G. Wang, P. N. Ross, C. A. Lucas, et al., Science 315, 493 (2007).
A. Ceylan, K. Jastrzembski, and S. I. Shah, Metall. Mater. Trans. A 37, 2033 (2006).
N. Toshima, H. Yan, and Y. Shiraishi, in Metal Nanoclusters in Catalysis and Materials Science: The Issue of Size Control, Ed. by B. Corain, G. Schmid, and N. Toshima (Elsevier, Amsterdam, 2007), p. 49.
J. Li, H. He, C. Hu, and J. Zhao, Frontiers Environ. Sci. Eng. 7, 302 (2013).
F. Tao, M. E. Grass, Y. Zhang, D. R. Butcher, J. R. Renzas, Z. Liu, J. Y. Chung, B. S. Mun, M. Salmeron, and G. A. Somorjai, Science 322, 932 (2008).
E. González, J. Arbiol, and V. F. Puntes, Science 334, 1377 (2011).
B. Lim, M. Jiang, P. H. Camargo, E. C. Cho, J. Tao, X. Lu, Y. Zhu, and Y. Xia, Science 324, 1302 (2009).
D. Lahiri, B. Bunker, B. Mishra, Z. Zhang, D. Meisel, C. Doudna, M. Bertino, F. D. Blum, A. Tokuhiro, and S. Chattopadhyay, J. Appl Phys. 97, 094304 (2005).
G. Allaedini, S. M. Tasirin, and P. Aminayi, J. Alloys Compd. 647, 809 (2015).
G. Allaedini, P. Aminayi, S. M. Tasirin, and E. Mahmoudi, Fullerenes, Nanotubes Carbon Nanostruct. 23, 968 (2015).
S. W. Han, Y. Kim, and K. Kim, J. Colloid Interface Sci. 208, 272 (1998).
G. Allaedini, S. M. Tasirin, M. Talib, M. Zainal, P. Aminayi, and I. Puspasari, Appl. Mech. Mater. 719, 132 (2015).
G. Allaedini, S. Tasirin, and P. Aminayi, Int. Nano Lett. 5, 183 (2015).
E. Abbasi, M. Milani, S. Fekri Aval, M. Kouhi, A. Akbarzadeh, H. Tayefi Nasrabadi, P. Nikasa, S.W. Joo, Y. Hanifehpour, K. Nejati-Koshki, and M. Samiei, Crit. Rev. Microbiol. 42, 173 (2016).
X. Wang, F. Song, Q. Chen, T. Wang, J. Wang, P. Liu, M. Shen, J. Wan, G. Wang, and J.-B. Xu, J. Am. Chem. Soc. 132, 6492 (2010).
P. Weyrich, W. Hölderich, and W. Sachtler, Appl. Catal. A: Gen. 163, 31 (1997).
Z. Ma, J. Yu, and S. Dai, Adv. Mater. 22, 261 (2010).
G. Allaedini, P. Aminayi, and S. M. Tasirin, J. Nanomater. 501, 961231 (2015).
G. Allaedini, S. Tasirin, P. Aminayi, Z. Yaakob, and M. Talib, React. Kinet., Mech. Catal. 116, 1 (2015).
A. Parker, J. R. Inst. Chem. 83, 349 (1959).
B. Coq and F. Figueras, J. Mol. Catal. A: Chem. 173, 117 (2001).
G. Allaedini, S. M. Tasirin, J. Sahari, M. Talib, and M. Zainal, Adv. Mater. Res. 2014, 148 (2014).
J. Plšek and Z. Bastl, J. Catal. 299, 109 (2013).
S. B. Rathod, A. B. Gambhire, B. R. Arbad, and M. K. Lande, ChemInform 41 (30), i (2010).
T.-X. Liu, X.-z. Li, and F.-b. Li, Chem. Eng. J. 157, 475 (2010).
A.-W. Xu, Y. Gao, and H.-Q. Liu, J. Catal. 207, 151 (2002).
Z. Shi and L. Jin, J. Non-Cryst. Solids 355, 213 (2009).
A. Phuruangrat, N. Ekthammathat, B. Kuntalue, P. Dumrongrojthanath, S. Thongtem, and T. Thongtem, J. Nanomater. 2014, (2014).
X. Yao, T. Liu, X. Liu, and L. Lu, Chem. Eng. J. 255, 28 (2014).
G.-R. Li, X.-H. Lu, W.-X. Zhao, C.-Y. Su, and Y.-X. Tong, Cryst. Growth Des. 8, 1276 (2008).
S.-Y. Kuo, W.-C. Chen, F.-I. Lai, C.-P. Cheng, H.-C. Kuo, S.-C. Wang, and W.-F. Hsieh, J. Cryst. Growth 287, 78 (2006).
S. M. Seyed and M. Parivash, Res. J. Chem. Environ. 17, 101 (2013).
A. V. Rosario, W. A. Christinelli, R. N. Barreto, and E. C. Pereira, J. Sol-Gel Sci. Technol. 64, 734 (2012).
N. Pal and A. Bhaumik, Adv. Colloid Interface Sci. 189, 21 (2013).
V. c. Štengl and S. Bakardjieva, J. Phys. Chem. C 114, 19308 (2010).
L. G. Devi and G. Krishnamurthy, J. Phys. Chem. A 115, 460 (2010).
N. Taguchi, A. Iwase, N. Maeda, T. Kojima, R. Taniguchi, S. Okuda, T. Akita, T. Abe, T. Kambara, and H. Ryuto, Rad. Phys. Chem. 78, 1049 (2009).
H. Zhang, J. Okuni, and N. Toshima, J. Colloid Interface Sci. 354, 131 (2011).
S. Salimah, J. Chem. Eng. Process Technol. 3, 122 (2012).
W. Kuang, Y. Fan, and Y. Chen, Catal. Lett. 50, 31 (1998).
Y. Zheng, K. Liu, H. Qiao, Y. Zhang, Y. Song, M. Yang, Y. Huang, N. Guo, Y. Jia, and H. You, Cryst EngComm 13, 1786 (2011).
Y. Sun, X. Hu, C. Y. Jimmy, Q. Li, W. Luo, L. Yuan, W. Zhang, and Y. Huang, Energy Environ. Sci. 4, 2870 (2011).
H. F. McMurdie, M. C. Morris, E. H. Evans, B. Paretzkin, W. Wong-Ng, L. Ettlinger, and C. R. Hubbard, Powder Diffract. 1, 64 (1986).
A. Chithambararaj, D. B. Mathi, N. R. Yogamalar, and A. C. Bose, Mater. Res. Express 2, 055004 (2015).
R. Ahmed, R. Jamil, and M. S. Ansari, IOP Conf. Ser.: Mater. Sci. Eng., 012044 (2014).
Y. Wang, L. Zhang, S. Li, and P. Jena, J. Phys. Chem. C 113, 9210 (2009).
A. Malik, S. Hameed, M. J. Siddiqui, M. M. Haque, and M. Muneer, Int. J. Photoenergy 2013, 9 (2013).
A. George, S. K. Sharma, S. Chawla, M. Malik, and M. Qureshi, J.Alloys Compd. 509, 5942 (2011).
J. R. Sohn, E. W. Chun, and Y. I. Pae, Bull. Korean Chem. Soc. 24, 1785 (2003).
Y.-J. Lin, Y.-H. Chang, W.-D. Yang, and B.-S. Tsai, J. Non-Cryst. Solids 352, 789 (2006).
B. M. Reddy, I. Ganesh, E. P. Reddy, A. Fernández, and P. G. Smirniotis, J. Phys. Chem. B 105, 6227 (2001).
N. F. Djaja and R. Saleh, Mater. Sci. Appl. 4, 145 (2013).
S. Kalpagam and T. Kannadasan, J. Chem., Biol. Phys. Sci. 4, 9 (2014).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Allaedini, G., Tasirin, S.M. & Aminayi, P. The effects of cerium doping concentration on the properties and photocatalytic activity of bimetallic Mo/Ce catalyst. Russ. J. Phys. Chem. 90, 2080–2088 (2016). https://doi.org/10.1134/S0036024416080094
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024416080094