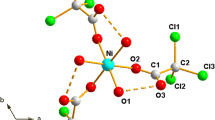
Abstract
The thermal decomposition of acidic cobalt carboxylates (ACCs) with unsaturated carboxylic anions has been studied by TG/DTA in combination with DSC and mass spectrometry of gaseous thermolysis products. The temperature ranges of the main ACC thermal decomposition stages have been determined as (1) 50–225°С for dehydration (2) 210–450°C for dehydrated carboxylate polymerization, and (3) 370–500°C for decarboxylation. It has been concluded that the listed stages may superimpose on one another and occur simultaneously.





Similar content being viewed by others
REFERENCES
A. Kh. Al’ Khazradzhi, A. V. Krylov, M. V. Kulikova, et al., Tonkie Khim. Tekhnol. 11, 28 (2016).
A. D. Pomogailo, A. S. Rozenberg, and G. I. Dzhardimalieva, Russ. Chem. Rev. 80, 257 (2011). https://doi.org/10.1070/RC2011v080n03ABEH004079
A. D. Pomogailo and G. I. Dzhardimalieva, Metal Polymer Hybrid Nanocomposites (Nauka, Moscow, 2015) [in Russian].
Y. Li, X.-Y. Yang, Y. Feng, et al., Critical Rev. Solid State Mater. Sci. 37, 1 (2012). https://doi.org/10.1080/10408436.2011.606512
A.-S. Malik, M. J. Duncan, and P. G. Bruce, J. Mater. Chem. 13, 2123 (2003).
R. L. Frost, W. Martens, and M. O. Adebajo, J. Therm. Anal. Calom. 81, 351 (2005).
A. Y. Ali-Mohamed, J. Coord. Chem. 29, 233 (1993).
F. T. Chuk, M. Koleva, and S. Mikhailova, Farmatsiya 38, 16 (1988).
K. M. Alam, F. M. Kaniz, and A. Gulzar, Pak. J. Sci. Ind. Res. 30, 707 (1987).
W. W. Yu, L. Qu, W. Guo, and X. Peng, Chem. Mater. 15, 2854 (2003).
W. W. Yu, Y. A. Wang, and X. Peng, Chem. Mater. 15, 4300 (2003).
B. S. Randhawa, H. Kaur, H. S. Dosanjh, and J. Singh, Ceram. Int. 42, 8891 (2016). https://doi.org/10.1016/j.ceramint.2016.02.142
F. Marandi, L. Hashemi, A. Morsali, and H. Krautscheid, Ultrason. Sonochem. 32, 86 (2016). https://doi.org/10.1016/j.ultsonch.2016.02.022
A. Saritha, B. Raju, D. N. Rao, et al., Adv. Powder Technol. 26, 349 (2015). https://doi.org/10.1016/j.apt.2014.11.005
S.-C. Liu, Z. F. Yue, and Y. Liu, J. Porous Mater. 22, 465 (2015). https://doi.org/10.1007/s10934-015-9915-y
B. Donkova and G. Avdeev, J. Therm. Anal. Calorim. 121, 567 (2015). https://doi.org/10.1007/s10973-015-4590-4
K. O. Abdulwahab, M. A. Malik, P. O’Brien, and G. A. Timco, Mater. Sci. Semiconductor Process. 27, 303 (2014). https://doi.org/10.1016/j.mssp.2014.06.052
D. Saikia, P. K. Saikia, P. K. Gogoi, et al., Mater. Chem. Phys. 131, 223 (2011). https://doi.org/10.1016/j.matchemphys.2011.09.011
S. A. Semenov, D. V. Drobot, V. Yu. Musatova, et al., Russ. J. Inorg. Chem. 60, 897 (2015). https://doi.org/10.1134/S0036023615080161
A. D. Pomogailo, G. I. Dzhardimalieva, and A. S. Rozenberg, J. Nanoparticle Res. 5, 497 (2003). https://doi.org/10.1023/B:NANO.0000006091.92638.a5
L. I. Yudanova, V. A. Logvinenko, and L. A. Sheludyakova, Russ. J. Inorg. Chem. 53, 1459 (2008). https://doi.org/10.1134/S0036023608090180
V. A. Shershnev, G. I. Dzhardimalieva, and D. P. Kiryukhin, Russ. Chem. Bull. 62, 1649 (2013). https://doi.org/10.1007/s11172-013-0239-2
K. Van Werde, D. Mondelaers, G. Vanhoyland, et al., J. Mater. Sci. 37, 81 (2002).
D. P. Domonov, S. I. Pechenyuk, A. N. Gosteva, et al., Vestn. YuUrGU, Ser. Khim. 6, 5 (2014).
S. A. Semenov, V. Yu. Musatova, D. V. Drobot, and G. I. Dzhardimalieva, Russ. J. Inorg. Chem. 63, 1217 (2018). https://doi.org/10.1134/S0036023618090164
N. P. Porollo, Z. G. Aliev, G. I. Dzhardimalieva, et al., Izv. Akad. Nauk, Ser. Khim., No. 2, 375 (1997).
A. S. Rozenberg, E. I. Aleksandrova, G. I. Dzhardimalieva, et al., Akad. Nauk, Ser. Khim., No. 10, 1743 (1993).
E. I. Aleksandrova, G. I. Dzhardimalieva, A. S. Rozenberg, and A. D. Pomogailo, Izv. Akad. Nauk, Ser. Khim., No. 2, 308 (1993).
N. Greenwood and A. Earnshaw, Chemistry of the Elements (Butterworth-Heinemann, Oxford, 1997; Binom Laboratoriya Znanii, Moscow, 2008).
G. B. Sergeev, Nanochemistry (Moscow, 2007) [in Russian].
N. N. Volkova, G. I. Dzhardimalieva, B. E. Krisyuk, et al., Russ. Chem. Bull. 65, 2025 (2016). https://doi.org/10.1007/s11172-016-1547-0
Funding
This work was financially supported by the Russian Foundation for Basic Research (project nos. 13-03-00342 and 19-03-00237).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests.
Additional information
Translated by E. Glushachenkova
Rights and permissions
About this article
Cite this article
Semenov, S.A., Musatova, V.Y., Drobot, D.V. et al. Thermal Decomposition of Acidic Cobalt(II) Carboxylates with Unsaturated Dicarboxylic Anions. Russ. J. Inorg. Chem. 65, 61–68 (2020). https://doi.org/10.1134/S0036023620010143
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023620010143