Abstract
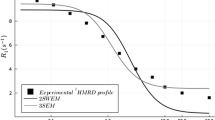
IR spectroscopy was used to study the rate of hydrogen-deuterium (H-D) exchange of peptide NH atoms in different forms of human hemoglobin (Hb) at pH 5–10 and temperatures of 10–63°C. The pH dependence of the H-D exchange rate fits the EX2 mechanism. At 10–30°C, there are two pH-dependent conformers of liganded Hb forms, the fluctuation probability being lower for the alkaline conformer. The differences between the conformers disappear at 40°C, where a third conformer, with a higher probability of local fluctuations, appears. Deoxyhemoglobin has no pH-dependent conformers in the pH range 6–9 at 20°C, and the probability of local fluctuations is considerably decreased compared to the acid conformer of liganded Hb. The destabilization of the liganded Hb structure by decreasing the pH to 5.0 at 20°C or increasing the temperature to 50–60°C at pH 7.1 enhances global fluctuations of the native structure ensuring the H-D exchange of slowly exchanging NH atoms. The mechanisms of local and high-temperature global fluctuations, as well as the possible similarity between the two pH-dependent conformers of liganded Hb and its functional R and R2 states revealed by X-ray analysis and NMR spectroscopy, are discussed.
Similar content being viewed by others
References
Sinha N., Smith-Gill S.J. 2002. Protein structure to function via dynamics. Prot. Pept. Lett. 9, 367–377.
Abaturov L.V. 1983. Thermal motion of protein: Smallscale fluctuations and conformational substates. Mol. Biol. 17, 693–704.
Abaturov L.V., Lebedev Yu.O., Nosova N.G., Shlyapnikov S.V. 1999. Dynamic structure of crystalline and dissolved ribonuclease A: Two main types of intramolecular motility. Mol. Biol. 33, 630–640.
Abaturov L.V., Lebedev Yu.O., Nosova N.G., Shlyapnikov S.V. 1999. Crystallographic temperature B factors and intramolecular motility of ribonuclease A. Mol. Biol. 33, 814–824.
Harata K., Kanai R. 2002. Crystallographic dissection of the thermal motion of protein-sugar complex. Proteins. 48, 53–62.
Bruschehweiler R. 2003. New approaches to the dynamic interpretation and prediction of NMR relaxation data from proteins. Curr. Opin. Struct. Biol. 13, 175–183.
Volkman B.F., Alam S.L., Satterlee J.D., Markley J.L. 1998. Solution structure and backbone dynamics of component IV Glycera dibranchiata monomeric hemoglobin-CO. Biochemistry. 37, 10906–10919.
Grivtsov A.G., Malenkov G.G., Abaturov L.V. 1983. Numerical modeling of the molecular dynamics of proteins. Mol. Biol. 17, 587–615.
Baldwin J., Chothia C. 1979. Hemoglobin: The structural changes related to ligand binding and its allosteric mechanism. J. Mol. Biol. 129, 175–220.
Case D.A., Karplus M.J. 1979. Dynamics of ligand binding to heme proteins. J. Mol. Biol. 132, 343–368.
Arrington C.B., Robertson A.D. 2000. Kinetics and thermodynamics of conformational equilibria in native proteins by hydrogen exchange. Meth. Enzymol. 323, 104–124.
Cavagnero S., Theriault Y., Narula S.S., Dyson H.J., Wright P.E. 2000. Amide proton hydrogen exchange rates for sperm whale myoglobin obtained from 15N-1H NMR spectra. Protein Sci. 9, 186–193.
Willumsen L. 1971. Hydrogen isotope exchange in the study of protein conformation. Compt. Rend. Trav. Lab. Carlsberg. 38, 223–295.
Abaturov L.V. 1976. Hydrogen exchange in proteins. Usp. Nauki Tekh. Ser. Mol. Biol. Moscow: VINITI. 8, 7–126.
Barksdale A.D, Rosenberg A. 1982. Acquisition and interpretation of hydrogen exchange data from peptides, polymers, and proteins. Meth. Biochem. Anal. 28, 1–113.
Abaturov L.V., Jinoria K.Sh., Varshavsky Ya.M., Yakobashvily N.N. 1977. Effect of ligand and heme on conformational stability (intramolecular conformational motility) of hemoglobin as revealed by hydrogen exchange. FEBS Lett. 77, 103–106.
Abaturov L.V., Lebedev Yu.O., Nosova N.G. 1983. Dynamic structure of globular proteins: Conformational rigidity and fluctuational motility. Mol. Biol. 17, 543–567.
Abaturov L.V., Yakobashvily N.N., Jinoria K.Sh., Molchanova T.P., Varshavsky Ya.M. 1976. Effect of intersubunit contact on intramolecular motility (conformational stability) of hemoglobin as revealed by hydrogen exchange. FEBS Lett. 70, 127–130.
Englander J.J., Mar C.D., Li W., Englander S.W., Kim J.S., Stranz D.D., Hamuro Y., Woods V.L., Jr. 2003. Protein structure change studied by hydrogen-deuterium exchange, functional labeling, and mass spectrometry. Proc. Natl. Acad. Sci. USA. 100, 7057–7062.
Huyghues-Despointes B.M.P., Scholtz J.M., Pace C.N. 1999. Protein conformational stabilities can be determined from hydrogen exchange rates. Nature Struct. Biol. 6, 910–912.
Bernstein F.C., Koetzle T.F., Williams G.J.B., Meyer E.F., Brice M.D., Rodergs J.R., Kennard O., Shimanouchi T., Tasumi M. 1977. The protein Data Bank: A computer-based archival file for macromolecular structures. J. Mol. Biol. 112, 535–542.
Hvidt A., Nielsen S.O. 1966. Hydrogen exchange in proteins. Adv. Protein Chem. 21, 287–386.
Bai Y., Milne J.S., Mayne L., Englander S.W. 1993. Primary structure effects on peptide group hydrogen exchange. Proteins. 17, 75–86.
Hedlund B.E., Hallaway P.E., Hallaway B.E., Benson E.S., Rosenberg A. 1978. Hydrogen exchange kinetics of human hemoglobins. The pH-dependence of solvent accessibility in cyanomet-, oxy-, and deoxyhemoglobin. J. Biol. Chem. 253, 3702–3707.
Müller R.G., Schmid K. 1983. Enthalpy of denaturation for human hemoglobin in the oxygenated and deoxygenated state. Thermochim. Acta. 69, 115–125.
Zhang Z., Smith D.L. 1996. Thermal-induced unfolding domains in aldolase identified by amide hydrogen exchange and mass spectrometry. Protein Sci. 5, 1282–1289.
Hiller R., Zhou Z.H., Adams M.W.W., Englander S.W. 1997. Stability and dynamics in a hyperthermophilic protein with melting temperature close to 200°C. Proc. Natl. Acad. Sci. USA. 94, 11329–11332.
Bai Y., Milne J.S., Mayne L., Englander S.W. 1994. Protein stability parameters measured by hydrogen exchange. Proteins. 20, 4–14.
Abaturov L.V., Lebedev Yu.O., Molchanova T.P., Nosova N.G., Faizullin D.A. 1990. Conformational rigidity and fluctuational motility of globular proteins: Water as a structure stabilizer and plasticizer. In: Ravnovesnaya dinamika struktury biopolimerov (Equilibrium Dynamics of Protein Structure). Eds. Burshtein E.A., Abaturov L.V. Pushchino: TsNTI, pp. 49–77.
Olsen K.W. 1994. Thermal denaturation procedure for hemoglobin. Meth. Enzymol. 231, 514–524.
Abaturov L.V., Ushakova M.M., Molchanova T.P., Yakobashvily N.N., Zhdanova K.I., Jinoria K.Sh. 1975. Comparative studies on the conformational stability and intramolecular motility of hemoglobin and its constituent parts. VII Int. Symp. Struct. und Function der Erythrozyten. Berlin: Academia-Verlag, 129–133.
Duan J., Nilsson L. 2005. Thermal unfolding simulation of a multimeric protein-transition state and unfolding pathways. Proteins. 59, 170–182.
Nakanishi M., Tsuboi M., Ikegami A. 1974. Fluctuation of the myoglobin structure. Bull. Chem. Soc. Japan. 47, 293–298.
Williams D.C., Jr., Benjamin D.C., Poljak R.J., Rule G.S. 1996. Global changes in amide hydrogen exchange rates for a protein antigen in complex with three different antibodies. J. Mol. Biol. 257, 866–876.
Arrington C.B., Robertson A.D. 2000. Microsecond to minute dynamics revealed by EX1-type hydrogen exchange at nearly every backbone hydrogen bond in a native protein. J. Mol. Biol. 296, 1307–1317.
Liem R.K.H., Calhoun D.B., Englander J.J., Englander S.W. 1980. A high energy structure change in hemoglobin studied by difference hydrogen exchange. J. Biol. Chem. 25, 10687–10694.
Malin E.L., Englander S.W. 1980. The slowest allosterically responsive hydrogens in hemoglobin. Completion of the hydrogen survey. J. Biol. Chem. 25, 10695–10701.
Mueser T.C., Rogers P.H., Arnone A. 2000. Interface sliding as illustrated by the multiple quaternary structures of liganded hemoglobin. Biochemistry. 39, 15353–15364.
Lukin J.A., Kontaxis G., Simplaceanu V., Yuan Y., Bax A., Ho C. 2003. Quaternary structure of hemoglobin in solution. Proc. Natl. Acad. Sci. USA. 100, 517–520.
Silva M.M., Rogers P.H., Arnone A. 1992. A third quaternary structure of human hemoglobin A at 1.7-Å resolution. J. Biol. Chem. 267, 17248–17256.
Briehl R.W., Hobbs J.F. 1970. Ultraviolet difference spectra in human hemoglobin: 1. Difference spectra in hemoglobin A and their relation to the function of hemoglobin. J. Biol. Chem. 245, 544–554.
Sawicki C.A., Gibson Q.H. 1976. Quaternary conformational changes in human hemoglobin studied by laser photolysis of carboxyhemoglobin. J. Biol. Chem. 251, 1533–1542.
McDonald M.J., Noble R.W. 1972. The effect of pH on the rates of ligand replacement reactions of human adult and fetal hemoglobins and their subunits. J. Biol. Chem. 247, 4282–4287.
Tian W.D., Sage J.T., Champion P.M. 1993. Investigations of ligand association and rates in the “open” and “closed” states of myoglobin. J. Mol. Biol. 233, 155–166.
Vendruscolo M., Paci J.T., Dobson C.M., Karplus M. 2003. Rare fluctuations of native proteins sampled by equilibrium hydrogen exchange. J. Am. Chem. Soc. 125, 15686–15687.
Chang C.-K., Simplaceanu V., Ho C. 2002. Effects of amino acid substitutions at β131 on the structure and properties of hemoglobin: Evidence for communication between α1β1-and α1β2-subunit interfaces. Biochemistry. 41, 5644–5655.
Yuan Y., Simplaceanu V., Lukin J.A., Ho C. 2002. NMR investigation of the dynamics of tryptophan side-chains in hemoglobins. J. Mol. Biol. 321, 863–878.
Eaton W.A., Henry E.R., Hofrichter J., Mozzarelly A. 1999. Is cooperative oxygen binding by hemoglobin really understood. Nature Struct. Biol. 6, 351–358.
Rujan I.N., Russu I.M. 2002. Allosteric effects of chloride ions at the intradimeric α1β1 and α1β2 interfaces of human hemoglobin. Proteins. 49, 413–419.
Mihailescu M.-R., Fronticelli C., Russu I.M. 2001. Allosteric free energy changes at the α1β2 interface of human hemoglobin probes by proton exchange of Trpβ37. Proteins. 44, 73–78.
Connelly G.P., McIntosh L.P. 1998. Characterization of a buried neutral histidine in Bacillus circulans xynalase: Internal dynamics and interaction with a bound water molecule. Biochemistry. 37, 1810–1818.
Author information
Authors and Affiliations
Additional information
Original Russian Text © L.V. Abaturov, N.G. Nosova, S.V. Shlyapnikov, D.A. Faizullin, 2006, published in Molekulyarnaya Biologiya, 2006, Vol. 40, No. 2, pp. 326–340.
Rights and permissions
About this article
Cite this article
Abaturov, L.V., Nosova, N.G., Shlyapnikov, S.V. et al. Conformational dynamics of the tetrameric hemoglobin molecule as revealed by hydrogen exchange: I. Effects of pH, temperature, and ligand binding. Mol Biol 40, 284–297 (2006). https://doi.org/10.1134/S0026893306020154
Received:
Issue Date:
DOI: https://doi.org/10.1134/S0026893306020154