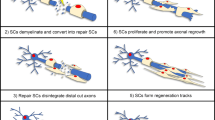
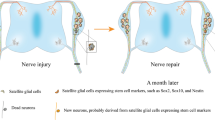
Abstract
The review addresses current concepts on the origin and functions of Schwann cells (SCs) as well as phenotypic characterization of their precursors at different ontogenetic stages. The necessity of versatile fundamental exploring SCs is dictated by searching for novel ways to stimulate the recovery of peripheral nerve fibers, including cell and gene therapy. Being a major structural component of the nerve, SCs have a decisive influence on degenerative and reparative processes therein. Particularly accentuated is the lack of knowledge of the molecular mechanisms that regulate SCs differentiation at different ontogenetic stages and their plasticity in the pathology of nerve conduction.
Similar content being viewed by others
References
Salzer, J.L., Schwann cell myelination, Cold Spring Harb. Perspect. Biol, 2015, vol. 7 (8), a020529. https://doi.org/10.1101/cshper-spect.a020529
Zalc, B., The acquisition of myelin: a success story, Novartis Found. Symp., 2006, vol.276, pp. 15–21.
Zalc, B., Goujet, D., and Colman, D., The origin of the myelination program in vertebrates, Curr. Biol, 2008, vol. 18 (12), pp. R511–R512. https://doi.org/10.1016/j.cub.2008.04.010
Salzer, J.L. and Zalc, B., Myelination, Curr. Biol, 206, vol. 26 (20), pp. R971-R975. https://doi.org/10.1016/j.cub.2016.07.074
Waller, A., New method for the study of the nervous system, Lond. J. Med., 1852, vol.4 (43), pp. 609–625.
Ramony Cajal, S., Degeneration and regeneration of the nervous system, 1–2, L., 1928, Oxf. H. Mil-ford.
Doynikov, B.S., Izbrannye trudypo neiromorfologii i nevropatologii. (The Selected Works on Neuromorphology and Neuropathology), 1955, Moscow.
Nozdrachev, A.D. and Chumasov, E.I., Perifericheskaya nervnaya sistema (Peripheral Nervous System), 1999, St. Petersburg.
Walsh, S. and Midha, R., Use of stem cells to augment nerve injury repair, Neurosurg., 2009, vol. 65, pp. 80–86.
Petrova, E.S., Studies of the histogenetic and neurodegenerative processes in the nervous system using heterotopic neurotransplantation, Morfol, 2009, vol. 36 (6), pp. 8–9.
Chelyshev, Yu.A., Regeneratsiya v nervnoi sisteme. Rukovodstvo po gistologii (Regeneration in the Nervous System. The Handbook on Histology), Danilov, R.K., Ed., 2011, St. Petersburg, pp. 656–665.
Petrova, E.S., The use of stem cells to stimulate regeneration of damaged nerve, Cytology, 2012, vol. 54, pp. 525–540.
Petrova, E.S., Injured nerve regeneration using cell-based therapies: current challenges, Acta Naturae, 2015, vol. 7 (3(26)), pp. 42–53.
Fairbairn, N.G., Meppelink, A.M., Ng-Glazier, J., Randolph, M.A., and Winograd, J.M., Augmenting peripheral nerve regeneration using stem cells: A review of current opinion, World J. Stem Cells, 2015, vol. 7 (1), pp. 11–26.
Shchanitsyn, I.N., Ivanov, A.N., Bazhanov, S.P., Ninel’, V.G., Puchinyan, D.M., and Norkin, I.A., Stimulation of peripheral nerve regeneration: current status, problems and perspectives, Usp. Fiziol. Nauk, 2017, vol. 48 (3), pp. 92–112.
Karagyaur, M.N., Makarevich, P.I., Shev-chenko, E.K., Stambolsky, D.V., Kalinina, N.I., and Parfyonova, Ye.V., Modern approaches to peripheral nerve regeneration after injury: the prospects of gene and cell therapy, Genes and Cells, 2017, vol. 12(1), pp. 6–14.
Schwann, T., Microscopical researches into the accordance in the structure and growth of animals and plants, London, 1847.
Bhatheja, K. and Field, J., Schwann cells: origins and role in axonal maintenance and regeneration, Int. J. Biochem. Cell Biol, 2006, vol. 38, pp. 1995–1999.
Pineda, A., The “lemmocyte” in peripheral-nerve tumors, J. Neurosurg., 1965, vol. 22, pp. 594–601.
Odinak, M.M., Zhivolupov, S.A., Rashi-dov, N.A., and Samartsev, I.N., Peculiarities of development of denervation-reinervation process in traumatic neuropathies and plexopathies, Vestn. Ross. Voenno-Med. Akad., 2007, vol.4 (20), pp. 130–140.
Terminologia histologica. Mezhdunarodnye terminy po tsitologii i gistologii cheloveka s oficial’nym spis-kom russkih ekvivalentov (Terminologia histologica. International Terms for Human Cytology and Histology with an official list of Russian Equivalents), Banin, V.V. and Bykov, V.L., Eds., 2009, Moscow.
Zochodne, D.W., Neurobiology of Peripheral Nerve Regeneration, Cambridge, New York, Melbourne, Madrid, Cape Town, Singapore, Sao Paulo, 2008.
Jessen, K.R., Mirsky, R., and Lloyd, A.C., Schwann cells: development and role in nerve repair, Cold Spring Harb. Perspect. Biol., 2015, vol.7 (7): a020487. https://doi.org/10.1101/csh-perspect.a020487
Taveggia, C., Zanazzi, G., Petrylak, A., Yano, H., Rosenbluth, J., Einheber, S., Xu, X., Esper, R.M., Loeb,J.A., Shrager, P., Chao, M.V., Falls, D.L., Role, L., and Salzer, J.L., Neuregulin-1 type III determines the ensheathment fate of axons, Neuron, 2005, vol. 47, pp. 681–694.
Jessen, K.R., Mirsky, R., and Lloyd, A.C., Schwann cells: development and role in nerve repair, Cold Spring Harb. Perspect. Biol, 2015, vol.7 (7), a020487. https://doi.org/10.1101/csh-perspect.a020487
Zalc, B., The acquisition of myelin: an evolutionary perspective, Brain Res., 206, vol. 1641 (Pt. A), pp. 4–10.
Monk, K.R., Feltri, M.L., and Taveggia, C., New insights on Schwann cell development, Glia, 2015, vol. 63, pp. 1376–1393.
Yu, W.M., Yu, H., Chen, Z.L., and Strickland, S., Disruption of laminin in the peripheral nervous system impedes nonmyelinating Schwann cell development and impairs nociceptive sensory function, Glia, 2009, vol. 57, pp. 850–859.
Griffin, J.W. and Thompson, W.J., Biology and pathology of nonmyelinating Schwann cells, Glia, 56, pp. 1518–1531.
Diamond, J., Holmes, M., and Coughlin, M., Endogenous NGF and nerve impulses regulate the collateral sprouting of sensory axons in the skin of the adult rat, J. Neurosci., 1992, vol. 12, pp. 1454–1466.
Young, P., Nie, J., Wang, X., McGlade, C.J., Rich, M.M., and Feng, G., LNX1 is a perisynaptic Schwann cell specific E3 ubiquitin ligase that interacts with ErbB2, Mol. Cell. Neurosci., 2005, vol. 30, pp. 238–248.
Zuo, Y., Lubischer, J.L., Kang, H., Tian, L., Mikesh, M., Marks, A., Scofield, V.L., Maika, S., Newman, C., Krieg, P., and Thompson, W.J., Fluorescent proteins expressed in mouse transgenic lines mark subsets of glia, neurons, macrophages, and dendritic cells for vital examination, J. Neurosci., 2004, vol. 24, pp. 10999–11009.
Smith, I.W., Mikesh, M., Lee, Y., and Thompson, W.J., Terminal Schwann cells participate in the competition underlying neuromuscular synapse elimination, J. Neurosci., 2013, vol. 33, pp. 17724–17736.
Kang, H., Tian, L., Mikesh, M., Lichtman, J.W., and Thompson, W.J., Terminal Schwann cells participate in neuromuscular synapse remodeling during reinnervation following nerve injury, J. Neurosci., 2014, vol. 34, pp. 6323–6333.
Le Douarin, N.M., Cell line segregation during peripheral nervous system ontogeny, Science, vol. 231, pp. 1515–1522.
Kidd, G.J., Ohno, N., and Trapp, B.D., Biology of Schwann cells, Handb. Clin. Neurol, 2013, vol. 115, pp. 55–79.
Liu, Z., Jin, Y.Q., Chen, L., Wang, Y., Yang, X., Cheng, J., Wu, W., Qi, Z., and Shen, Z., Specific marker expression and cell state of Schwann cells during culture in vitro, PLoS One, 2015, vol. 10 (4), e0123278. https://doi.org/10.1371/jour-nal.pone.0123278
Hartline, D.K. and Colman, D.R., Rapid conduction and the evolution of giant axons and myelinated fibers, Curr. Biol., 2007, vol. 17 (1), pp. R29–R35. https://doi.org/10.1016/j.cub.2006.11.042
De Bellard, M.E., Myelin in cartilaginous fish, Brain Res., 2016, vol. 1641 (Pt. A), pp. 34–42.
Hartline, D.K., The evolutionary origins of glia, Glia, 2011, vol. 59, pp. 1215–1236.
Davis, A.D., Weatherby, T.M., Hartline, D.K., and Lenz, P.H., Myelin-like sheaths in copepod axons, Nature, 1999, vol. 398, p. 571.
Kastriti, M.E. and Adameyko, I., Specification, plasticity and evolutionary origin of peripheral glial cells, Curr. Opin. Neurobiol., 2017, vol. 47, pp. 196–202.
Birchmeier, C., ErbB receptors and the development of the nervous system, Exp. Cell Res., 2009, vol. 315, pp. 611–618.
Riethmacher, D., Sonnenberg-Riethmacher, E., Brinkmann, V., Yamaai, T., Lewin, G.R., and Birchmeier, C., Severe neuropathies in mice with targeted mutations in the ErbB3 receptor, Nature, 1997, vol. 389, pp. 725–730.
Nave, K.-A and Trapp, B.D., Axon-glial signaling and the glial support of axon function, Annu. ReNeurosci., 2008, vol. 31, pp. 535–561.
Varon, S.S. and Bunge, R.P., Trophic mechanisms in the peripheral nervous system, Annu. ReNeurosci., 1978, vol. 1, pp. 327–361.
Morrison, B.M., Lee, Y., and Rothstein, J.D., Oligodendroglia: metabolic supporters of axons, Trends Cell Biol, 2013, vol. 23, pp. 644–651.
Viader, A, Sasaki, Y, Kim, S., Strickland, A., Workman, C.S., Yang, K., Gross, R.W., and Milbrandt, J., Aberrant Schwann cell lipid metabolism linked to mitochondrial deficits leads to axon degeneration and neuropathy, Neuron, 2013, vol. 77, pp. 886–898.
Court, F.A., Midha, R., Cisterna, B.A., Grochmal, J., Shakhbazau, A., Hendriks, W.T., and Van Minnen, J., Morphological evidence for a transport of ribosomes from Schwann cells to regenerating axons, Glia, 2011, vol. 59, pp. 1529–1539.
Ching, R.C. and Kingham, P.J., The role of exosomes in peripheral nerve regeneration, Neural Regen. Res., 2015, vol. 10, pp. 743–747.
Lee, Y., El Andaloussi, S., and Wood, M.J., Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy, Human Mol Gen., 2012, vol. 21 (Rl), pp. R125–R134. https://doi.org/10.1093/hmg/dds317.2012
Lopez-Leal, R. and Court, F.A., Schwann cell exosomes mediate neuron-glia communication and enhance axonal regeneration, Cell Mol. Neurobiol, 2016, vol. 36, pp. 429–436.
Heumann, R., Regulation of the synthesis of nerve growth factor, J. Exp. Biol, 1987, vol. 132, pp. 133–150.
Lewin, G.R. and Barde, Y.A., Physiology of the neurotrophins, Annu. ReNeurosci., 1996, vol. 19, pp. 289–317.
Chen, Z.L., Yu, W.M., and Strickland, S., Peripheral regeneration, Annu. ReNeurosci., 2007, vol. 30, pp. 209–233.
Jiang, X., Liu, L., Zhang, B., Lu, Z., Qiao, L., Feng, X., and Yu, W., Effects of Angelica extract on Schwann cell proliferation and expressions of related proteins, Evid. Based Compl. Altern. Med., 2017, 6358392. https://doi.org/10.1155/2017/6358392.
Shamash, S., Reichert, F., and Rotshenker, S., The cytokine network of Wallerian degeneration: tumor necrosis factoralpha, interleukin-1 alpha, and interleukin-lbeta, J. Neurosci., 2002, vol. 22, pp. 3052–3060.
Tofaris, G.K., Patterson, P.H., Jessen, K.R., and Mirsky, R., Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF, J. Neurosci., 2002, vol. 22, pp. 6696–6703.
Chen, P., Piao, X., and Bonaldo, P., Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury, Acta Neuropathol., 2015, vol. 130, pp. 605–618.
Jung, J., Frump, D., Su, J., Wang, W., Mozaffar, T., and Gupta, R., Desert hedgehog is a mediator of demyelination in compression neuropathies, Exp. Neurol., 2015, vol. 271, 84–94.
Li, W., Kohara, H., Uchida, Y., James, J.M., Soneji, K., Cronshaw, D.G., Zou, Y.R., Nagasawa, T., and Mukouyama, Y.S., Peripheral nerve-derived CXCL12 and VEGF-A regulate the patterning of arterial vessel branching in developing limb skin, DeCell, 2013, vol. 24, pp. 359–371.
Hirata, K. and Kawabuchi, M., Myelin phagocytosis by macrophages and nonmacrophages during Wallerian degeneration, Microsc. Res. Tech., 2002, vol. 57, pp. 541–547.
Beuche, W. and Friede, R.L., The role of non-resident cells in Wallerian degeneration, J. Neurocy-tol, 1984, vol. 13, pp. 767–796.
Chumasov, E.I. and Svetikova, K.M., The structure and nature of the macrophages participating in Wallerian degeneration of nerve fibers, Arkh. Anat. Gistol. Embriol, 1991, vol. 100 (5), pp. 13–21.
Brosius Lutz, A. and Barres, B.A, Contrasting the glial response to axon injury in the central and peripheral nervous systems, DeCell, 2014, vol. 28, pp. 7–17.
Perry, V.H., Tsao, J.W., Fearn, S., and Brown, M.C., Radiation-induced reductions in macrophage recruitment have only slight effects on myelin degeneration in sectioned peripheral nerves of mice, Eur. J. Neurosci., 1995, vol. 7, pp. 271–280.
Gomez-Sanchez, J.A, Carty, L., Iruarrizaga-Lejarreta, M., Palomo-Irigoyen, M., Varela-Rey, M., Griffith, M., Hantke, J., Macias-Camara, N., Azkargorta, M., Aurrekoetxea, I., De Juan, V.G., Jefferies, H.B., Aspichueta, P., Elortza, F., Aransay, AM., Martinez-Chantar, M.L., Baas, F., Mato, J.M., Mirsky, R., Woodhoo, A, and Jessen, K.R., Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves, J. Cell Biol., 2015, vol. 210, pp. 153–168.
Brosius Lutz, A, Chung, W.S., Sloan, S.A., Carson, G.A., Zhou, L., Lovelett, E., Posada, S., Zuchero, J.B., and Barres, B.A., Schwann cells use TAM receptor-mediated phagocytosis in addition to autophagy to clear myelin in a mouse model of nerve injury, Proc. Natl. Acad. Sci. USA, 2017, vol. 114 (38), E8072–E8080. https://doi.org/10.1073/pnas.1710566114
Camara-Lemarroy, C.R., Guzman-de la Garza, F.J., and Fernandez-Garza, N.E., Molecular inflammatory mediators in peripheral nerve degeneration and regeneration, Neuroimmuno-modul, 2010, vol. 17, pp. 314–324.
Arthur-Farraj, P.J., Latouche, M., Wilton, D.K., Quintes, S., Chabrol, E., Banerjee, A., Woodhoo, A, Jenkins, B., Rahman, M., Turmaine, M., Wicher, G.K., Mitter, R., Greensmith, L., Behrens, A., Raivich, G., Mirsky, R., and Jessen, K.R., C-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration, Neuron, 2012, vol. 75, pp. 633–647.
Gomez-Sanchez, J.A., Pilch, K.S., van der Lans, M., Fazal, S.V., Benito, C., Wagstaff, L.J., Mirsky, R., and Jessen, K.R., After nerve injury, lineage tracing shows that myelin and Remak Schwann cells elongate extensively and branch to form repair Schwann cells, which shorten radically on remyelination, J. Neurosci., 2017, vol. 37, pp. 9086–9099.
Carr, M.J. and Johnston, A.P., Schwann cells as drivers of tissue repair and regeneration, Curr. Opin. Neurobiol, 2017, vol. 47, pp. 52–57.
Kaukua, N., Shahidi, M.K., Konstantinidou, C., Dyachuk, V., Kaucka, M., Furlan, A., An, Z., Wang, L., Hultman, I., Ahrlund-Richter, L., Blom, H., Brismar, H., Lopes, N.A., Pachnis, V., Suter, U., Clevers, H., Thesleff, I., Sharpe, P., Ernfors, P., Fried, K., and Adameyko, I., Glial origin of mesenchymal stem cells in a tooth model system, Nature, 2014, vol. 513, pp. 551–554.
Adameyko, I., Lallemend, F., Aquino, J.B., Pereira, J.A, Topilko, P., Muller, T., Fritz, N., Beljajeva, A, Mochii, M., Liste, I., Usoskin, D., Suter, U., Birchmeier, C., and Ernfors, P., Schwann cell precursors from nerve innervation are a cellular origin of melanocytes in skin, Cell, 2009, vol. 139, pp. 366–379.
Widera, D., Hermann, P., Zander, C., Imiel-ski, Y., Heidbreder, M., Heilemann, M., Kalts-chmidt, C., and Kaltschmidt, B., Schwann cells can be reprogrammed to multipotency by culture, Stem Cells Dev., 2011, vol. 20, pp. 2053–2064.
Uesaka, T., Nagashimada, M., and Enomoto, H., Neuronal Differentiation in Schwann cell lineage underlies postnatal neurogenesis in the enteric nervous system, J. Neurosci., 2015, vol. 35, pp. 9879–9888.
Tapinos, N. and Rambukkana, A., Insights into regulation of human Schwann cell proliferation by Erkl/2 via a MEK-independent and p56Lck-dependent pathway from leprosy bacilli, Proc. Natl. Acad. Sci. USA, 2005, vol. 102, pp. 9188–9193.
Wippold, F.J. 2nd, Lubner, M., Perrin, R.J., Lammle, M., and Perry, A, Neuropathology for the neuroradiologist: Antoni A and Antoni B tissue patterns, AJNR Am. J. Neuroradiol, 2007, vol. 28 (9), pp. 1633–1638.
Gomez-Sanchez, J.A., Lopez de Armentia, M., Lujan, R., Kessaris, N., Richardson, W.D., and Cabedo, H., Sustained axon-glial signaling induces Schwann cell hyperproliferation, Remak bundle myelination, and tumorigenesis, J. Neurosci., 2009, vol. 29, pp. 11304–11315.
Nenashev, E.A., Cherekaev, V.A., Kadasheva, A.B., Kozlov, A.V., Rotin, D.L., and Stepanyan, M.A, Transformation of trigeminal nerve tumor into malignant peripheral nerve sheath tumor (MPNST), Vopr. Neirokhirurg. im. N.N. Burdenko, 2012, vol. 76 (5), pp. 58–62.
Chelyshev, J.A and Viktorov, I.V., Cell technologies of remyelination after spinal cord trauma, Nevrol. Vestnik, 2009, vol. 41 (1), pp. 49–55.
Levi, A.D., Gunard, V., Aebischer, P., and Bunge, R.P., The functional characteristics of Schwann cells cultured from human peripheral nerve after transplantation into a gap within the rat sciatic nerve, J. Neurosci., 1994, vol. 14 (3), Pt. 1, pp. 1309–1319.
Kim, D.H., Connolly, S.E., Kline, D.G., Voorhies, R.M., Smith, A., Powell, M., Yoes, T., and Daniloff, J.K., Labeled Schwann cell transplants versus sural nerve grafts in nerve repair, J. Neurosurg., 1994, vol. 80, pp. 254–260.
Rajangam, T. and An, S.S., Fibrinogen and fibrin based micro and nano scaffolds incorporated withdrugs, proteins, cells and genes for therapeutic biomedical applications, Int. J. Nanomed., 2013, vol. 8, pp. 3641–3662.
Hadlock, T., Sundback, C., Hunter, D., Cheney, M., and Vacanti, J.P., A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration, Tissue Eng., 2000, vol. 6, pp. 119–127.
Radtke, C., Akiyama, Y., Lankford, K.L., Vogt, P.M., Krause, D.S., and Kocsis, J.D., Integration of engrafted Schwann cells into injured peripheral nerve: axonal association and nodal formation on regenerated axons, Neurosci. Lett., 2005, vol. 387, pp. 85–89.
Schmitte, R., Tipold, A, Stein, V.M., Schenk, H., Flieshardt, C., Grothe, C., and Haastert, K., Genetically modified canine Schwann cells: in vitro and in vivo evaluation of their suitability for peripheral nerve tissue engineering, J. Neurosci. Methods, 2010, vol. 186, pp. 202–208.
Timmer, M., Robben, S., Miiller-Ostermeyer, F., Nikkhah, G., and Grothe, C., Axonal regeneration across long gaps in silicone chambers filled with Schwann cells overexpressing high molecular weight FGF-2, Cell Transplant., 2003, vol. 12, pp. 265–277.
Haastert, K., Lipokatic, E., Fischer, M., Timmer, M., and Grothe, C., Differentially promoted peripheral nerve regeneration by grafted Schwann cells over-expressing different FGF-2 isoforms, Neurobiol. Dis., 2006, vol. 21, pp. 138–153.
Li, Q., Ping, P., Jiang, H., and Liu, K., Nerve conduit filled with GDNF gene-modified Schwann cells enhances regeneration of the peripheral nerve, Microsurg., 2006, vol.26, 116–121.
Pettingill, L.N., Minter, R.L., and Shepherd, R.K., Schwann cells genetically modified to express neurotrophins promote spiral ganglion neuron survival in vitro, Neurosci., 2008, vol. 152, pp. 821–818.
Madduri, S. and Gander, B., Schwann cell delivery of neurotrophic factors for peripheral nerve regeneration, J. Periph. Nerv. Syst., 2010, vol. 15, pp. 93–103.
Shaimardanova, G.F., Bashankaev, S.D., Izmailov, A.A., Fadeyev, F.O., Sokolov, M.Ye., and Islamov, R.R., Stimulation of regeneration of rat spinal cord by adenoviruses carrying genes encoding GDNF, NCAM1 AND VEGF165, Morfol, 2017, vol. 151 (3), p. 115.
Shaimardanova, G.F., Mukhamedshina, Y.O., and Chelyshev, Y.A., Assessment of efficiency of local delivery pathways of therapeutic genes in murine spinal cord injury: a correlation of structural and functional parameters, Sovr. Tekhnol. Med., 2013, vol. 5 (3), pp. 16–22.
Petrova, E.S., A study of regeneration of the crushed rat sciatic nerve after use of experimental cell therapy, Mezhdunar. Nauchno-Issled. Zh., 2018, vol. 4 (70), pp. 42–45.
Petrova, E.S., Isaeva, E.N., Kolos, E.A, and Korzhevskii, D.E., Vascularization of the damaged nerve under the effect of experimental cell therapy, Klet. Tekhnol. Biol. Med., 2018, vol. 1, pp. 53–57.
Funding
This work was implemented within a state assignment by the Russian Federation Ministry of Education and Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study did not involve human or animal subjects as research objects.
Additional information
Russian Text © The Author(s), 2019, published in Zhurnal Evolyutsionnoi Biokhimii i Fiziologii, 2019, Vol. 55, No. 6, pp. 383–397.
Rights and permissions
About this article
Cite this article
Petrova, E.S. Current Views on Schwann Cells: Development, Plasticity, Functions. J Evol Biochem Phys 55, 433–447 (2019). https://doi.org/10.1134/S0022093019060012
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093019060012