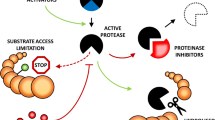
Abstract
Each plant genome encodes hundreds of proteolytic enzymes. These enzymes can be divided into five distinct classes: cysteine-, serine-, aspartic-, threonine-, and metalloproteinases. Despite the differences in their structural properties and activities, members of all of these classes in plants are involved in the processes of regulated cell death–a basic feature of eukaryotic organisms. Regulated cell death in plants is an indispensable mechanism supporting plant development, survival, stress responses, and defense against pathogens. This review summarizes recent advances in studies of plant proteolytic enzymes functioning in the initiation and execution of distinct types of regulated cell death.
Similar content being viewed by others
Abbreviations
- ER:
-
endoplasmic reticulum
- NCCD:
-
Nomenclature Committee on Cell Death
- PCD:
-
programmed cell death
- PS-SCL:
-
positional scanning substrate combinatorial library
- RCD:
-
regulated cell death
- VPE:
-
vacuolar processing enzyme.
References
Galluzzi, L., Bravo-San Pedro, J. M., Vitale, I., Aaronson, S. A., Abrams, J. M., Adam, D., Alnemri, E. S., Altucci, L., Andrews, D., Annicchiarico-Petruzzelli, M., Baehrecke, E. H., Bazan, N. G., Bertrand, M. J., Bianchi, K., Blagosklonny, M. V., Blomgren, K., Borner, C., Bredesen, D. E., Brenner, C., Campanella, M., Candi, E., Cecconi, F., Chan, F. K., Chandel, N. S., Cheng, E. H., Chipuk, J. E., Cidlowski, J. A., Ciechanover, A., Dawson, T. M., Dawson, V. L., De Laurenzi, V., De Maria, R., Debatin, K. M., Di Daniele, N., Dixit, V. M., Dynlacht, B. D., El-Deiry, W. S., Fimia, G. M., Flavell, R. A., Fulda, S., Garrido, C., Gougeon, M. L., Green, D. R., Gronemeyer, H., Hajnoczky, G., Hardwick, J. M., Hengartner, M. O., Ichijo, H., Joseph, B., Jost, P. J., Kaufmann, T., Kepp, O., Klionsky, D. J., Knight, R. A., Kumar, S., Lemasters, J. J., Levine, B., Linkermann, A., Lipton, S. A., Lockshin, R. A., Lopez-Otin, C., Lugli, E., Madeo, F., Malorni, W., Marine, J. C., Martin, S. J., Martinou, J. C., Medema, J. P., Meier, P., Melino, S., Mizushima, N., Moll, U., Munoz-Pinedo, C., Nunez, G., Oberst, A., Panaretakis, T., Penninger, J. M., Peter, M. E., Piacentini, M., Pinton, P., Prehn, J. H., Puthalakath, H., Rabinovich, G. A., Ravichandran, K. S., Rizzuto, R., Rodrigues, C. M., Rubinsztein, D. C., Rudel, T., Shi, Y., Simon, H. U., Stockwell, B. R., Szabadkai, G., Tait, S. W., Tang, H. L., Tavernarakis, N., Tsujimoto, Y., Vanden Berghe, T., Vandenabeele, P., Villunger, A., Wagner, E. F., Walczak, H., White, E., Wood, W. G., Yuan, J., Zakeri, Z., Zhivotovsky, B., Melino, G., and Kroemer, G. (2015) Essential versus accessory aspects of cell death: recommendations of the NCCD 2015, Cell Death Differ., 22, 58–73.
Lockshin, R. A. (2008) Early work on apoptosis, an interview with Richard Lockshin, Cell Death Differ., 15, 1091–1095.
Kroemer, G., El-Deiry, W. S., Golstein, P., Peter, M. E., Vaux, D., Vandenabeele, P., Zhivotovsky, B., Blagosklonny, M. V., Malorni, W., Knight, R. A., Piacentini, M., Nagata, S., and Melino, G. (2005) Nomenclature Committee on Cell Death. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death, Cell Death Differ., 12, 1463–1467.
Galluzzi, L., Vitale, I., Abrams, J. M., Alnemri, E. S., Baehrecke, E. H., Blagosklonny, M. V., Dawson, T. M., Dawson, V. L., El-Deiry, W. S., Fulda, S., Gottlieb, E., Green, D. R., Hengartner, M. O., Kepp, O., Knight, R. A., Kumar, S., Lipton, S. A., Lu, X., Madeo, F., Malorni, W., Mehlen, P., Nuñez, G., Peter, M. E., Piacentini, M., Rubinsztein, D. C., Shi, Y., Simon, H. U., Vandenabeele, P., White, E., Yuan, J., Zhivotovsky, B., Melino, G., and Kroemer, G. (2012) Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012, Cell Death Differ., 19, 107–120.
Crawford, E. D., and Wells, J. A. (2011) Caspase substrates and cellular remodeling, Annu. Rev. Biochem., 80, 1055–1087.
Van Doorn, W. G., Beers, E. P., Dangl, J. L., Franklin-Tong, V. E., Gallois, P., Hara-Nishimura, I., Jones, A. M., Kawai-Yamada, M., Lam, E., Mundy, J., Mur, L. A., Petersen, M., Smertenko, A., Taliansky, M., Van Breusegem, F., Wolpert, T., Woltering, E., Zhivotovsky, B., and Bozhkov, P. V. (2011) Morphological classification of plant cell deaths, Cell Death Differ., 18, 1241–1246.
Sanchez-Vallet, A., Mesters, J. R., and Thomma, B. P. (2015) The battle for chitin recognition in plant−microbe interactions, FEMS Microbiol. Rev., 39, 171–183.
Solovieva, A. D., Frolova, O. Yu., Solovyev, A. G., Morozov, S. Yu., and Zamyatnin, A. A., Jr. (2013) Effect of mitochondria-targeted antioxidant SkQ1 on programmed cell death induced by viral proteins in tobacco plants, Biochemistry (Moscow), 78, 1006–1012.
Lukhovitskaya, N. I., Yelina, N. E., Zamyatnin, A. A., Jr., Schepetilnikov, M. V., Solovyev, A. G., Sandgren, M., Morozov, S. Y., Valkonen, J. P., and Savenkov, E. I. (2005) Expression, localization and effects on virulence of the cys-teine-rich 8 kDa protein of Potato mop-top virus, J. Gen. Virol., 86, 2879–2889.
Ye, C. M., Chen, S., Payton, M., Dickman, M. B., and Verchot, J. (2013) TGBp3 triggers the unfolded protein response and SKP1-dependent programmed cell death, Mol. Plant Pathol., 14, 241–255.
Petrov, V., Hille, J., Mueller-Roeber, B., and Gechev, T. S. (2015) ROS-mediated abiotic stress-induced programmed cell death in plants, Front. Plant Sci, 6, 69.
Shahid, M., Pourrut, B., Dumat, C., Nadeem, M., Aslam, M., and Pinelli, E. (2014) Heavy-metal-induced reactive oxygen species: phytotoxicity and physicochemical changes in plants, Rev. Environ. Contam. Toxicol., 232, 1–44.
Danon, A., Rotari, V. I., Gordon, A., Mailhac, N., and Gallois, P. (2004) Ultraviolet-C overexposure induces pro-grammed cell death in Arabidopsis, which is mediated by caspase-like activities and which can be suppressed by cas-pase inhibitors, p35 and defender against apoptotic death, J. Biol. Chem., 279, 779–787.
Kim, Y., Wang, M., Bai, Y., Zeng, Z., Guo, F., Han, N., Bian, H., Wang, J., Pan, J., and Zhu, M. (2014) Bcl-2 sup-presses activation of VPEs by inhibiting cytosolic Ca2+ level with elevated K+ efflux in NaCl-induced PCD in rice, Plant Physiol. Biochem., 80, 168–175.
Li, Z., Yue, H., and Xing, D. (2012) MAP kinase 6-medi-ated activation of vacuolar processing enzyme modulates heat shock-induced programmed cell death in Arabidopsis, New Phytol., 195, 85–96.
Bagniewska-Zadworna, A., Arasimowicz-Jelonek, M., Smolinski, D. J., and Stelmasik, A. (2015) New insights into pioneer root xylem development: evidence obtained from Populus trichocarpa plants grown under field condi-tions, Ann. Bot., 113, 1235–1247.
Avci, U., Petzold, H. E., Ismail, I. O., Beers, E. P., and Haigler, C. H. (2008) Cysteine proteases XCP1 and XCP2 aid micro-autolysis within the intact central vacuole during xylogenesis in Arabidopsis roots, Plant J., 56, 303–315.
Del Pozo, O., and Lam, E. (1998) Caspases and pro-grammed cell death in the hypersensitive response of plants to pathogens, Curr. Biol., 8, 1129–1132.
Bonneau, L., Ge, Y., Drury, G. E., and Gallois, P. (2008) What happened to plant caspases? J. Exp. Bot., 59, 491–499.
Sasaki, T. (1998) The rice genome project in Japan, Proc. Natl. Acad. Sci. USA, 95, 2027–2028.
Dennis, C., and Surridge, C. (2000) Arabidopsis thaliana genome. Introduction, Nature, 408, 791.
Hatsugai, N., Kuroyanagi, M., Yamada, K., Meshi, T., Tsuda, S., Kondo, M., Nishimura, M., and Hara-Nishimura, I. (2004) A plant vacuolar protease, VPE, mediates virus-induced hypersensitive cell death, Science, 305, 855–858.
Rojo, E., Martin, R., Carter, C., Zouhar, J., Pan, S., Plotnikova, J., Jin, H., Paneque, M., Sanchez-Serrano, J. J., Baker, B., Ausubel, F. M., and Raikhel, N. V. (2004) VPEgamma exhibits a caspase-like activity that contributes to defense against pathogens, Curr. Biol., 14, 1897–1906.
Rawlings, N. D., Waller, M., Barrett, A. J., and Bateman, A. (2014) MEROPS: the database of proteolytic enzymes, their substrates and inhibitors, Nucleic Acids Res., 42, 503–509.
Hiraiwa, N., Nishimura, M., and Hara-Nishimura, I. (1999) Vacuolar processing enzyme is self-catalytically activated by sequential removal of the C-terminal and N-terminal propeptides, FEBS Lett., 447, 213–216.
Kuroyanagi, M., Nishimura, M., and Hara-Nishimura, I. (2002) Activation of Arabidopsis vacuolar processing enzyme by self-catalytic removal of an auto-inhibitory domain of the C-terminal propeptide, Plant Cell Physiol., 43, 143–151.
Hara-Nishimura, I., and Hatsugai, N. (2011) The role of vacuole in plant cell death, Cell Death Differ., 18, 1298–1304.
Hatsugai, N., Yamada, K., Goto-Yamada, S., and Hara-Nishimura, I. (2015) Vacuolar processing enzyme in plant programmed cell death, Front. Plant Sci., 6, 234.
Hara-Nishimura, I., Hatsugai, N., Nakaune, S., Kuroyanagi, M., and Nishimura, M. (2005) Vacuolar pro-cessing enzyme: an executor of plant cell death, Curr. Opin. Plant Biol., 8, 404–408.
Kuroyanagi, M., Yamada, K., Hatsugai, N., Kondo, M., Nishimura, M., and Hara-Nishimura, I. (2005) Vacuolar processing enzyme is essential for mycotoxin-induced cell death in Arabidopsis thaliana, J. Biol. Chem., 280, 32914–32920.
Gauthier, A., Lamotte, O., Reboutier, D., Bouteau, F., Pugin, A., and Wendehenne, D. (2007) Cryptogein-induced anion effluxes: electrophysiological properties and analysis of the mechanisms through which they contribute to the elicitor-triggered cell death, Plant Signal. Behav., 2, 86–95.
Kumar, D., Rampuria, S., Singh, N. K., Shukla, P., and Kirti, P. B. (2015) Characterization of a vacuolar process-ing enzyme expressed in Arachis diogoi in resistance responses against late leaf spot pathogen, Phaeoisariopsis personata, Plant Mol. Biol., 88, 177–191.
Qiang, X., Zechmann, B., Reitz, M. U., Kogel, K. H., and Schafer, P. (2012) The mutualistic fungus Piriformospora indica colonizes Arabidopsis roots by inducing an endoplas-mic reticulum stress-triggered caspase-dependent cell death, Plant Cell, 24, 794–809.
Deng, M., Bian, H., Xie, Y., Kim, Y., Wang, W., Lin, E., Zeng, Z., Guo, F., Pan, J., Han, N., Wang, J., Qian, Q., and Zhu, M. (2011) Bcl-2 suppresses hydrogen peroxide-induced programmed cell death via OsVPE2 and OsVPE3, but not via OsVPE1 and OsVPE4, in rice, FEBS J., 278, 4797–4810.
Kadono, T., Tran, D., Errakhi, R., Hiramatsu, T., Meimoun, P., Briand, J., Iwaya-Inoue, M., Kawano, T., and Bouteau, F. (2010) Increased anion channel activity is an unavoidable event in ozone-induced programmed cell death, PLoS One, 5, e13373.
Yakimova, E. T., Kapchina-Toteva, V. M., Laarhoven, L. J., Harren, F. M., and Woltering, E. J. (2006) Involvement of ethylene and lipid signaling in cadmium-induced pro-grammed cell death in tomato suspension cells, Plant Physiol. Biochem., 44, 581–589.
Yakimova, E. T., Kapchina-Toteva, V. M., and Woltering, E. J. (2007) Signal transduction events in aluminum-induced cell death in tomato suspension cells, J. Plant Physiol., 164, 702–708.
Kariya, K., Demiral, T., Sasaki, T., Tsuchiya, Y., Turkan, I., Sano, T., Hasezawa, S., and Yamamoto, Y. (2013) A novel mechanism of aluminum-induced cell death involving vac-uolar processing enzyme and vacuolar collapse in tobacco cell line BY-2, J. Inorg. Biochem., 128, 196–201.
Nakaune, S., Yamada, K., Kondo, M., Kato, T., Tabata, S., Nishimura, M., and Hara-Nishimura, I. (2005) A vacuolar processing enzyme, deltaVPE, is involved in seed coat for-mation at the early stage of seed development, Plant Cell, 17, 876–887.
Radchuk, V., Weier, D., Radchuk, R., Weschke, W., and Weber, H. (2011) Development of maternal seed tissue in barley is mediated by regulated cell expansion and cell dis-integration and coordinated with endosperm growth, J. Exp. Bot., 62, 1217–1227.
Tran, V., Weier, D., Radchuk, R., Thiel, J., and Radchuk, V. (2014) Caspase-like activities accompany programmed cell death events in developing barley grains, PLoS One, 9, e109426.
Kinoshita, T., Yamada, K., Hiraiwa, N., Kondo, M., Nishimura, M., and Hara-Nishimura, I. (1999) Vacuolar processing enzyme is up-regulated in the lytic vacuoles of vegetative tissues during senescence and under various stressed conditions, Plant J., 19, 43–53.
Muller, G. L., Drincovich, M. F., Andreo, C. S., and Lara, M. V. (2010) Role of photosynthesis and analysis of key enzymes involved in primary metabolism throughout the lifespan of the tobacco flower, J. Exp. Bot., 61, 3675–3688.
Uren, A. G., O’Rourke, K., Aravind, L. A., Pisabarro, M. T., Seshagiri, S., Koonin, E. V., and Dixit, V. M. (2000) Identification of paracaspases and metacaspases: two ancient families of caspase-like proteins, one of which plays a key role in MALT lymphoma, Mol. Cell, 6, 961–967.
Vercammen, D., Van de Cotte, B., De Jaeger, G., Eeckhout, D., Casteels, P., Vandepoele, K., Vandenberghe, I., Van Beeumen, J., Inze, D., and Van Breusegem, F. (2004) Type II metacaspases Atmc4 and Atmc9 of Arabidopsis thaliana cleave substrates after arginine and lysine, J. Biol. Chem., 279, 45329–45336.
Watanabe, N., and Lam, E. (2005) Two Arabidopsis meta-caspases AtMCP1b and AtMCP2b are arginine/lysine-spe-cific cysteine proteases and activate apoptosis-like cell death in yeast, J. Biol. Chem., 280, 14691–14699.
Bozhkov, P. V., Suarez, M. F., Filonova, L. H., Daniel, G., Zamyatnin, A. A., Jr., Rodriguez-Nieto, S., Zhivotovsky, B., and Smertenko, A. (2005) Cysteine protease mcII-Pa executes programmed cell death during plant embryogene-sis, Proc. Natl. Acad. Sci. USA, 102, 14463–14468.
Tsiatsiani, L., Van Breusegem, F., Gallois, P., Zavialov, A., Lam, E., and Bozhkov, P. V. (2011) Metacaspases, Cell Death Differ., 18, 1279–1288.
Acosta-Maspons, A., Sepulveda-Garcia, E., Sanchez-Baldoquin, L., Marrero-Gutierrez, J., Pons, T., Rocha-Sosa, M., and Gonzalez, L. (2014) Two aspartate residues at the putative p10 subunit of a type II metacaspase from Nicotiana tabacum L. may contribute to the substrate-bind-ing pocket, Planta, 239, 147–160.
Fagundes, D., Bohn, B., Cabreira, C., Leipelt, F., Dias, N., Bodanese-Zanettini, M. H., and Cagliari, A. (2015) Caspases in plants: metacaspase gene family in plant stress responses, Funct. Integr. Genom., 15, 639–649.
Choi, C. J., and Berges, J. A. (2013) New types of metacas-pases in phytoplankton reveal diverse origins of cell death proteases, Cell Death Dis., 4, e490.
Wen, S., Ma, Q. M., Zhang, Y. L., Yang, J. P., Zhao, G. H., Fu, D. Q., Luo, Y. B., and Qu, G. Q. (2013) Biochemical evidence of key residues for the activation and autoprocess-ing of tomato type II metacaspase, FEBS Lett., 587, 2517–2522.
Watanabe, N., and Lam, E. (2011) Calcium-dependent activation and autolysis of Arabidopsis metacaspase 2d, J. Biol. Chem., 286, 10027–10040.
Zhang, Y., and Lam, E. (2011) Sheathing the swords of death: post-translational modulation of plant metacaspas-es, Plant Signal. Behav., 6, 2051–2056.
Belenghi, B., Romero-Puertas, M. C., Vercammen, D., Brackenier, A., Inze, D., Delledonne, M., and Van Breusegem, F. (2007) Metacaspase activity of Arabidopsis thaliana is regulated by S-nitrosylation of a critical cysteine residue, J. Biol. Chem., 282, 1352–1358.
Huang, L., Zhang, H., Hong, Y., Liu, S., Li, D., and Song, F. (2015) Stress-responsive expression, subcellular localiza-tion and protein–protein interactions of the rice metacas-pase family, Int. J. Mol. Sci., 16, 16216–16241.
Bollhoner, B., Zhang, B., Stael, S., Denance, N., Overmyer, K., Goffner, D., Van Breusegem, F., and Tuominen, H. (2013) Post mortem function of AtMC9 in xylem vessel elements, New Phytol., 200, 498–510.
Tsiatsiani, L., Timmerman, E., De Bock, P. J., Vercammen, D., Stael, S., Van de Cotte, B., Staes, A., Goethals, M., Beunens, T., Van Damme, P., Gevaert, K., and Van Breusegem, F. (2013) The Arabidopsis metacaspase 9 degradome, Plant Cell, 25, 2831–2847.
Coll, N. S., Vercammen, D., Smidler, A., Clover, C., Van Breusegem, F., Dangl, J. L., and Epple, P. (2010) Arabidopsis type I metacaspases control cell death, Science, 330, 1393–1397.
Coll, N. S., Smidler, A., Puigvert, M., Popa, C., Valls, M., and Dangl, J. L. (2014) The plant metacaspase AtMC1 in pathogen-triggered programmed cell death and aging: functional linkage with autophagy, Cell Death Differ., 21, 1399–1408.
Kim, S. M., Bae, C., Oh, S. K., and Choi, D. (2013) A pepper (Capsicum annuum L.) metacaspase 9 (Camc9) plays a role in pathogen-induced cell death in plants, Mol. Plant Pathol., 14, 557–566.
Wang, X., Wang, X., Feng, H., Tang, C., Bai, P., Wei, G., Huang, L., and Kang, Z. (2012) TaMCA4, a novel wheat metacaspase gene functions in programmed cell death induced by the fungal pathogen Puccinia striiformis f. sp. tritici, Mol. Plant Microbe Interact., 25, 755–764.
Hao, L., Goodwin, P. H., and Hsiang, T. (2007) Expression of a metacaspase gene of Nicotiana benthamiana after inoc-ulation with Colletotrichum destructivum or Pseudomonas syringae pv. tomato, and the effect of silencing the gene on the host response, Plant Cell Rep., 26, 1879–1888.
Watanabe, N., and Lam, E. (2011) Arabidopsis metacaspase 2d is a positive mediator of cell death induced during biot-ic and abiotic stresses, Plant J., 66, 969–982.
He, R., Drury, G. E., Rotari, V. I., Gordon, A., Willer, M., Farzaneh, T., Woltering, E. J., and Gallois, P. (2008) Metacaspase-8 modulates programmed cell death induced by ultraviolet light and H2O2 in Arabidopsis, J. Biol. Chem., 283, 774–783.
Minina, E. A., Filonova, L. H., Fukada, K., Savenkov, E. I., Gogvadze, V., Clapham, D., Sanchez-Vera, V., Suarez, M. F., Zhivotovsky, B., Daniel, G., Smertenko, A., and Bozhkov, P. V. (2013) Autophagy and metacaspase deter-mine the mode of cell death in plants, J. Cell Biol., 203, 917–927.
Wrzaczek, M., Vainonen, J. P., Stael, S., Tsiatsiani, L., Help-Rinta-Rahko, H., Gauthier, A., Kaufholdt, D., Bollhoner, B., Lamminmaki, A., Staes, A., Gevaert, K., Tuominen, H., Van Breusegem, F., Helariutta, Y., and Kangasjarvi, J. (2015) GRIM REAPER peptide binds to receptor kinase PRK5 to trigger cell death in Arabidopsis, EMBO J., 34, 55–66.
Vercammen, D., Belenghi, B., Van de Cotte, B., Beunens, T., Gavigan, J. A., De Rycke, R., Brackenier, A., Inze, D., Harris, J. L., and Van Breusegem, F. (2006) Serpin1 of Arabidopsis thaliana is a suicide inhibitor for metacaspase 9, J. Mol. Biol., 364, 625–636.
Sundstrom, J. F., Vaculova, A., Smertenko, A. P., Savenkov, E. I., Golovko, A., Minina, E., Tiwari, B. S., Rodriguez-Nieto, S., Zamyatnin, A. A., Jr., Valineva, T., Saarikettu, J., Frilander, M. J., Suarez, M. F., Zavialov, A., Stahl, U., Hussey, P. J., Silvennoinen, O., Sundberg, E., Zhivotovsky, B., and Bozhkov, P. V. (2009) Tudor staphylococcal nucle-ase is an evolutionarily conserved component of the pro-grammed cell death degradome, Nat. Cell Biol., 11, 1347–1354.
Caudy, A. A., Ketting, R. F., Hammond, S. M., Denli, A. M., Bathoorn, A. M., Tops, B. B., Silva, J. M., Myers, M. M., Hannon, G. J., and Plasterk, R. H. (2003) A micro-coccal nuclease homologue in RNAi effector complexes, Nature, 425, 411–414.
Scadden, A. D. (2005) The RISC subunit Tudor-SN binds to hyper-edited double-stranded RNA and promotes its cleavage, Nat. Struct. Mol. Biol., 12, 489–496.
Hundley, H. A., and Bass, B. L. (2010) ADAR editing in double-stranded UTRs and other noncoding RNA sequences, Trends Biochem. Sci., 35, 377–383.
Zamyatnin, A. A., Jr., Lyamzaev, K. G., and Zinovkin, R. A. (2010) A-to-I RNA editing: a contribution to diversity of the transcriptome and an organism’s development, Biochemistry (Moscow), 75, 1316–1323.
Gutierrez-Beltran, E., Moschou, P. N., Smertenko, A. P., and Bozhkov, P. V. (2015) Tudor staphylococcal nuclease links formation of stress granules and processing bodies with mRNA catabolism in Arabidopsis, Plant Cell, 27, 926–943.
Strobel, I., and Osiewacz, H. D. (2013) Poly(ADP-ribose) polymerase is a substrate recognized by two metacaspases of Podospora anserine, Eukaryot. Cell, 12, 900–912.
Martinez, M., Cambra, I., Gonzalez-Melendi, P., Santamaria, M. E., and Diaz, I. (2012) C1A cysteine-pro-teases and their inhibitors in plants, Physiol. Plant., 145, 85–94.
Trobacher, C. P., Senatore, A., and Greenwood, J. S. (2006) Masterminds or minions? Cysteine proteinases in plant programmed cell death, Can. J. Bot., 84, 651–667.
Turk, V., Turk, B., and Turk, D. (2001) Lysosomal cysteine proteases: facts and opportunities, EMBO J., 20, 4629–4633.
Than, M. E., Helm, M., Simpson, D. J., Lottspeich, F., Huber, R., and Gietl, C. (2004) The 2.0 Å crystal structure and substrate specificity of the KDEL-tailed cysteine endopeptidase functioning in programmed cell death of Ricinus communis endosperm, J. Mol. Biol., 336, 1103–1116.
Richau, K. H., Kaschani, F., Verdoes, M., Pansuriya, T. C., Niessen, S., Stuber, K., Colby, T., Overkleeft, H. S., Bogyo, M., and Van der Hoorn, R. A. (2012) Subclassification and biochemical analysis of plant papain-like cysteine proteas-es displays subfamily-specific characteristics, Plant Physiol., 158, 1583–1599.
Hierl, G., Howing, T., Isono, E., Lottspeich, F., and Gietl, C. (2014) Ex vivo processing for maturation of Arabidopsis KDEL-tailed cysteine endopeptidase 2 (AtCEP2) pro-enzyme and its storage in endoplasmic reticulum derived organelles, Plant Mol. Biol., 84, 605–620.
Liu, H., Chen, L., Li, Q., Zheng, M., and Liu, J. (2014) Computational study on substrate specificity of a novel cys-teine protease 1 precursor from Zea mays, Int. J. Mol. Sci., 15, 10459–10478.
Savvateeva, L. V., Gorokhovets, N. V., Makarov, V. A., Serebryakova, M. V., Solovyev, A. G., Morozov, S. Y., Reddy, V. P., Zernii, E. Y., Zamyatnin, A. A., Jr., and Aliev, G. (2015) Glutenase and collagenase activities of wheat cysteine protease Triticain-α: feasibility for enzymatic ther-apy assays, Int. J. Biochem. Cell Biol., 62, 115–124.
Van der Hoorn, R. A. (2008) Plant proteases: from pheno-types to molecular mechanisms, Annu. Rev. Plant Biol., 59, 191–223.
Schmid, M., Simpson, D., Kalousek, F., and Gietl, C. (1998) A cysteine endopeptidase with a C-terminal KDEL motif isolated from castor bean endosperm is a marker enzyme for the ricinosome, a putative lytic compartment, Planta, 206, 466–475.
Schmid, M., Simpson, D. J., Sarioglu, H., Lottspeich, F., and Gietl, C. (2001) The ricinosomes of senescing plant tis-sue bud from the endoplasmic reticulum, Proc. Natl. Acad. Sci. USA, 98, 5353–5358.
Gu, C., Shabab, M., Strasser, R., Wolters, P. J., Shindo, T., Niemer, M., Kaschani, F., Mach, L., and Van der Hoorn, R. A. (2012) Post-translational regulation and trafficking of the granulin-containing protease RD21 of Arabidopsis thaliana, PLoS One, 7, e32422.
Madureira, H. C., Da Cunha, M., and Jacinto, T. (2006) Immunolocalization of a defense-related 87 kDa cystatin in leaf blade of tomato plants, Environ. Exp. Bot., 55, 201–208.
Martinez, M., and Diaz, I. (2008) The origin and evolution of plant cystatins and their target cysteine proteinases indi-cate a complex functional relationship, BMC Evol. Biol., 8, 198.
Nissen, M. S., Kumar, G. N., Youn, B., Knowles, D. B., Lam, K. S., Ballinger, W. J., Knowles, N. R., and Kang, C. (2009) Characterization of Solanum tuberosum multicys-tatin and its structural comparison with other cystatins, Plant Cell, 21, 861–875.
Green, A. R., Nissen, M. S., Kumar, G. N., Knowles, N. R., and Kang, C. (2013) Characterization of Solanum tuberosum multicystatin and the significance of core domains, Plant Cell, 25, 5043–5052.
Martinez, M., Diaz-Mendoza, M., Carrillo, L., and Diaz, I. (2007) Carboxy terminal extended phytocystatins are bifunctional inhibitors of papain and legumain cysteine proteinases, FEBS Lett., 581, 2914–2918.
Margis-Pinheiro, M., Zolet, A. C., Loss, G., Pasquali, G., and Margis, R. (2008) Molecular evolution and diversifica-tion of plant cysteine proteinase inhibitors: new insights after the poplar genome, Mol. Phylogenet. Evol., 49, 349–355.
Lampl, N., Budai-Hadrian, O., Davydov, O., Joss, T. V., Harrop, S. J., Curmi, P. M., Roberts, T. H., and Fluhr, R. (2010) Arabidopsis AtSerpin1, crystal structure and in vivo interaction with its target protease RESPONSIVE TO DESICCATION-21 (RD21), J. Biol. Chem., 285, 13550–13560.
Fluhr, R., Lampl, N., and Roberts, T. H. (2012) Serpin protease inhibitors in plant biology, Physiol. Plant., 145, 95–102.
Ahmed, S. U., Rojo, E., Kovaleva, V., Venkataraman, S., Dombrowski, J. E., Matsuoka, K., and Raikhel, N. V. (2000) The plant vacuolar sorting receptor AtELP is involved in transport of NH(2)-terminal propeptide-con-taining vacuolar proteins in Arabidopsis thaliana, J. Cell Biol., 149, 1335–1344.
Helm, M., Schmid, M., Hierl, G., Terneus, K., Tan, L., Lottspeich, F., Kieliszewski, M. J., and Gietl, C. (2008) KDEL-tailed cysteine endopeptidases involved in pro-grammed cell death, intercalation of new cells, and dis-mantling of extensin scaffolds, J. Bot., 95, 1049–1062.
Greenwood, J. S., Helm, M., and Gietl, C. (2005) Ricinosomes and endosperm transfer cell structure in pro-grammed cell death of the nucellus during Ricinus seed development, Proc. Natl. Acad. Sci. USA, 102, 2238–2243.
Howing, T., Huesmann, C., Hoefle, C., Nagel, M. K., Isono, E., Hückelhoven, R., and Gietl, C. (2014) Endoplasmic reticulum KDEL-tailed cysteine endopepti-dase 1 of Arabidopsis (AtCEP1) is involved in pathogen defense, Front. Plant Sci., 5, 58.
Hierl, G., Vothknecht, U., and Gietl, C. (2012) Programmed cell death in Ricinus and Arabidopsis: the function of KDEL cysteine peptidases in development, Physiol. Plant., 145, 103–113.
Zhang, D., Liu, D., Lv, X., Wang, Y., Xun, Z., Liu, Z., Li, F., and Lu, H. (2014) The cysteine protease CEP1, a key execu-tor involved in tapetal programmed cell death, regulates pollen development in Arabidopsis, Plant Cell, 26, 2939–2961.
Valpuesta, V., Lange, N. E., Guerrero, C., and Reid, M. S. (1995) Up-regulation of a cysteine protease accompanies the ethylene-insensitive senescence of daylily (Hemero-callis) flowers, Plant Mol. Biol., 28, 575–582.
Nadeau, J. A., Zhang, X. S., Li, J., and O’Neill, S. D. (1996) Ovule development: identification of stage-specific and tissue-specific cDNAs, Plant Cell, 8, 213–239.
Rocha, A. J., Soares, E. L., Costa, J. H., Costa, W. L., Soares, A. A., Nogueira, F. C., Domont, G. B., and Campos, F. A. (2013) Differential expression of cysteine peptidase genes in the inner integument and endosperm of developing seeds of Jatropha curcas L. (Euphorbiaceae), Plant Sci., 213, 30–37.
Trobacher, C. P., Senatore, A., Holley, C., and Greenwood, J. S. (2013) Induction of a ricinosomal-pro-tease and programmed cell death in tomato endosperm by gibberellic acid, Planta, 237, 665–679.
Senatore, A., Trobacher, C. P., and Greenwood, J. S. (2009) Ricinosomes predict programmed cell death leading to anther dehiscence in tomato, Plant Physiol., 149, 775–790.
Yamada, K., Matsushima, R., Nishimura, M., and Hara-Nishimura, I. (2001) A slow maturation of a cysteine pro-tease with a granulin domain in the vacuoles of senescing Arabidopsis leaves, Plant Physiol., 127, 1626–1634.
Bateman, A., and Bennett, H. P. (2009) The granulin gene family: from cancer to dementia, BioEssays, 31, 1245–1254.
Carter, C., Pan, S., Zouhar, J., Avila, E. L., Girke, T., and Raikhel, N. V. (2004) The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected pro-teins, Plant Cell, 16, 3285–3303.
Lampl, N., Alkan, N., Davydov, O., and Fluhr, R. (2013) Set-point control of RD21 protease activity by AtSerpin1 controls cell death in Arabidopsis, Plant J., 74, 498–510.
Zhao, P., Zhou, X. M., Zhang, L. Y., Wang, W., Ma, L. G., Yang, L. B., Peng, X. B., Bozhkov, P. V., and Sun, M. X. (2013) A bipartite molecular module controls cell death activation in the basal cell lineage of plant embryos, PLoS Biol., 11, e1001655.
Kim, M. J., Yamamoto, D., Matsumoto, K., Inoue, M., Ishida, T., Mizuno, H., Sumiya, S., and Kitamura, K. (1992) Crystal structure of papain–E64-c complex. Binding diversity of E64-c to papain S2 and S3 subsites, Biochem. J., 287, 797–803.
Zhao, C., Johnson, B. J., Kositsup, B., and Beers, E. P. (2000) Exploiting secondary growth in Arabidopsis. Construction of xylem and bark cDNA libraries and cloning of three xylem endopeptidases, Plant Physiol., 123, 1185–1196.
Funk, V., Kositsup, B., Zhao, C., and Beers, E. P. (2002) The Arabidopsis xylem peptidase XCP1 is a tracheary ele-ment vacuolar protein that may be a papain ortholog, Plant Physiol., 128, 84–94.
Petzold, H. E., Zhao, M., and Beers, E. P. (2012) Expression and functions of proteases in vascular tissues, Physiol. Plant., 145, 121–129.
McLellan, H., Gilroy, E. M., Yun, B. W., Birch, P. R., and Loake, G. J. (2009) Functional redundancy in the Arabidopsis cathepsin B gene family contributes to basal defense, the hypersensitive response and senescence, New Phytol., 183, 408–418.
Gilroy, E. M., Hein, I., van der Hoorn, R., Boevink, P. C., Venter, E., McLellan, H., Kaffarnik, F., Hrubikova, K., Shaw, J., Holeva, M., Lopez, E. C., Borras-Hidalgo, O., Pritchard, L., Loake, G. J., Lacomme, C., and Birch, P. R. (2007) Involvement of cathepsin B in the plant disease resistance hypersensitive response, Plant J., 52, 1–13.
Bernoux, M., Timmers, T., Jauneau, A., Briere, C., De Wit, P. J., Marco, Y., and Deslandes, L. (2008) RD19, an Arabidopsis cysteine protease required for RRS1-R-mediat-ed resistance, is relocalized to the nucleus by the Ralstonia solanacearum PopP2 effector, Plant Cell, 20, 2252–2264.
Xu, F. X., and Chye, M. L. (1999) Expression of cysteine proteinase during developmental events associated with programmed cell death in brinjal, Plant J., 17, 321–327.
Lohman, K. N., Gan, S., John, M. C., and Amasino, R. M. (1994) Molecular analysis of natural leaf senescence in Arabidopsis thaliana, Physiol. Plant., 92, 322–328.
Otegui, M. S., Noh, Y. S., Martinez, D. E., Vila Petroff, M. G., Staehelin, L. A., Amasino, R. M., and Guiamet, J. J. (2005) Senescence-associated vacuoles with intense proteolytic activity develop in leaves of Arabidopsis and soybean, Plant J., 41, 831–844.
Singh, S., Giri, M. K., Singh, P. K., Siddiqui, A., and Nandi, A. K. (2013) Down-regulation of OsSAG12-1 results in enhanced senescence and pathogen-induced cell death in transgenic rice plants, J. Biosci., 38, 583–592.
Marino, G., Uria, J. A., Puente, X. S., Quesada, V., Bordallo, J., and Lopez-Otin, C. (2003) Human autophagins, a family of cysteine proteinases potentially implicated in cell degrada-tion by autophagy, J. Biol. Chem., 278, 3671–3678.
Kaminskyy, V., and Zhivotovsky, B. (2012) Proteases in autophagy, Biochim. Biophys. Acta, 1824, 44–50.
Escamez, S., and Tuominen, H. (2014) Programs of cell death and autolysis in tracheary elements: when a suicidal cell arranges its own corpse removal, J. Exp. Bot., 65, 1313–1321.
Minina, E. A., Bozhkov, P. V., and Hofius, D. (2014) Autophagy as initiator or executioner of cell death, Trends Plant Sci., 19, 692–697.
Li, M., Hou, Y., Wang, J., Chen, X., Shao, Z. M., and Yin, X. M. (2011) Kinetics comparisons of mammalian Atg4 homologues indicate selective preferences toward diverse Atg8 substrates, J. Biol. Chem., 286, 7327–7338.
Woo, J., Park, E., and Dinesh-Kumar, S. P. (2014) Differential processing of Arabidopsis ubiquitin-like Atg8 autophagy proteins by Atg4 cysteine proteases, Proc. Natl. Acad. Sci. USA, 111, 863–868.
Chichkova, N. V., Shaw, J., Galiullina, R. A., Drury, G. E., Tuzhikov, A. I., Kim, S. H., Kalkum, M., Hong, T. B., Gorshkova, E. N., Torrance, L., Vartapetian, A. B., and Taliansky, M. (2010) Phytaspase, a relocalizable cell death promoting plant protease with caspase specificity, EMBO J., 29, 1149–1161.
Chichkova, N. V., Galiullina, R. A., Taliansky, M. E., and Vartapetian, A. B. (2008) Tissue disruption activates a plant caspase-like protease with TATD cleavage specifici-ty, Plant Stress, 2, 89–95.
Dodson, G., and Wlodawer, A. (1998) Catalytic triads and their relatives, Trends Biochem. Sci., 23, 347–352.
Rautengarten, C., Steinhauser, D., Bussis, D., Stintzi, A., Schaller, A., Kopka, J., and Altmann, T. (2005) Inferring hypotheses on functional relationships of genes: analysis of the Arabidopsis thaliana subtilase gene family, PLoS Comput. Biol., 1, e40.
Schaller, A., Stintzi, A., and Graff, L. (2012) Subtilases–ver-satile tools for protein turnover, plant development, and inter-actions with the environment, Physiol. Plant., 145, 52–66.
Yamagata, H., Masuzawa, T., Nagaoka, Y., Ohnishi, T., and Iwasaki, T. (1994) Cucumisin, a serine protease from melon fruits, shares structural homology with subtilisin and is generated from a large precursor, J. Biol. Chem., 269, 32725–32731.
Vartapetian, A. B., Tuzhikov, A. I., Chichkova, N. V., Taliansky, M., and Wolpert, T. J. (2011) A plant alternative to animal caspases: subtilisin-like proteases, Cell Death Differ., 18, 1289–1297.
Ottmann, C., Rose, R., Huttenlocher, F., Cedzich, A., Hauske, P., Kaiser, M., Huber, R., and Schaller, A. (2009) Structural basis for Ca2+-independence and activation by homodimerization of tomato subtilase 3, Proc. Natl. Acad. Sci. USA, 106, 17223–17228.
Chichkova, N. V., Galiullina, R. A., Beloshistov, R. E., Balakireva, A. V., and Vartapetian, A. B. (2014) Phytaspases: aspartate-specific proteases involved in plant cell death, Bioorg. Khim., 40, 658–664.
Galiullina, R. A., Kasperkiewicz, P., Chichkova, N. V., Szalek, A., Serebryakova, M. V., Poreba, M., Drag, M., and Vartapetian, A. B. (2015) Substrate specificity and possible heterologous targets of phytaspase, a plant cell death protease, J. Biol. Chem., 290, 24806–24815.
Poreba, M., Szalek, A., Kasperkiewicz, P., and Drag, M. (2014) Positional scanning substrate combinatorial library (PS-SCL) approach to define caspase substrate specificity, Methods Mol. Biol., 1133, 41–59.
Fomicheva, A. S., Tuzhikov, A. I., Beloshistov, R. E., Trusova, S. V., Galiullina, R. A., Mochalova, L. V., Chichkova, N. V., and Vartapetian, A. B. (2012) Programmed cell death in plants, Biochemistry (Moscow), 77, 1452–1464.
Chichkova, N. V., Kim, S. H., Titova, E. S., Kalkum, M., Morozov, V. S., Rubtsov, Y. P., Kalinina, N. O., Taliansky, M. E., and Vartapetian, A. B. (2004) A plant caspase-like protease activated during the hypersensitive response, Plant Cell, 16, 157–171.
Srivastava, R., Liu, J. X., and Howell, S. H. (2008) Proteolytic processing of a precursor protein for a growth-promoting peptide by a subtilisin serine protease in Arabidopsis, Plant J., 56, 219–227.
Coffeen, W. C., and Wolpert, T. J. (2004) Purification and characterization of serine proteases that exhibit caspase-like activity and are associated with programmed cell death in Avena sativa, Plant Cell, 16, 857–873.
Faro, C., and Gal, S. (2005) Aspartic proteinase content of the Arabidopsis genome, Curr. Prot. Pept. Sci., 6, 493–500.
Dunn, B. M. (2002) Structure and mechanism of the pepsin-like family of aspartic peptidases, Chem. Rev., 102, 4431–4458.
Geier, G., Banaj, H. J., Heid, H., Bini, L., Pallini, V., and Zwilling, R. (1999) Aspartyl proteases in Caenorhabditis elegans. Isolation, identification and characterization by a combined use of affinity chromatography, two-dimension-al gel electrophoresis, microsequencing and databank analysis, Eur. J. Biochem., 264, 872–879.
Guo, R., Xu, X., Carole, B., Li, X., Gao, M., Zheng, Y., and Wang, X. (2013) Genome-wide identification, evolu-tionary and expression analysis of the aspartic protease gene superfamily in grape, BMC Genom., 14, 554.
Niu, N., Liang, W., Yang, X., Jin, W., Wilson, Z. A., Hu, J., and Zhang, D. (2013) EAT1 promotes tapetal cell death by regulating aspartic proteases during male reproductive development in rice, Nat. Commun., 4, 1445.
Chen, F., and Foolad, M. R. (1997) Molecular organiza-tion of a gene in barley which encodes a protein similar to aspartic protease and its specific expression in nucellar cells during degeneration, Plant Mol. Biol., 35, 821–831.
Ge, X., Dietrich, C., Matsuno, M., Li, G., Berg, H., and Xia, Y. (2005) An Arabidopsis aspartic protease functions as an anti-cell-death component in reproduction and embryogenesis, EMBO Rep., 6, 282–288.
Phan, H. A., Iacuone, S., Li, S. F., and Parish, R. W. (2011) The MYB80 transcription factor is required for pollen development and the regulation of tapetal pro-grammed cell death in Arabidopsis thaliana, Plant Cell, 23, 2209–2224.
Kurepa, J., and Smalle, J. A. (2008) Structure, function and regulation of plant proteasomes, Biochimie, 90, 324–335.
Kurepa, J., Wang, S., Li, Y., and Smalle, J. (2009) Proteasome regulation, plant growth and stress tolerance, Plant Signal. Behav., 4, 924–927.
Han, J. J., Lin, W., Oda, Y., Cui, K. M., Fukuda, H., and He, X. Q. (2012) The proteasome is responsible for cas-pase-3-like activity during xylem development, Plant J., 72, 129–141.
Hatsugai, N., Iwasaki, S., Tamura, K., Kondo, M., Fuji, K., Ogasawara, K., Nishimura, M., and Hara-Nishimura, I. (2009) A novel membrane fusion-mediated plant immunity against bacterial pathogens, Genes Dev., 23, 2496–2506.
Maidment, J. M., Moore, D., Murphy, G. P., Murphy, G., and Clark, I. M. (1999) Matrix metalloproteinase homo-logues from Arabidopsis thaliana. Expression and activity, J. Biol. Chem., 274, 34706–34710.
Marino, G., Huesgen, P. F., Eckhard, U., Overall, C. M., Schroder, W. P., and Funk, C. (2014) Family-wide charac-terization of matrix metalloproteinases from Arabidopsis thaliana reveals their distinct proteolytic activity and cleavage site specificity, Biochem. J., 457, 335–346.
Hadler-Olsen, E., Fadnes, B., Sylte, I., Uhlin-Hansen, L., and Winberg, J. O. (2011) Regulation of matrix metallopro-teinase activity in health and disease, FEBS J., 278, 28–45.
Delorme, V. G., McCabe, P. F., Kim, D. J., and Leaver, C. J. (2000) A matrix metalloproteinase gene is expressed at the boundary of senescence and programmed cell death in cucumber, Plant Physiol., 123, 917–927.
Roberts, I. N., Caputo, C., Criado, M. V., and Funk, C. (2012) Senescence-associated proteases in plants, Physiol. Plant., 145, 130–139.
Diaz-Mendoza, M., Velasco-Arroyo, B., Gonzalez-Melendi, P., Martinez, M., and Diaz, I. (2014) C1A cys-teine protease–cystatin interactions in leaf senescence, J. Exp. Bot., 65, 3825–3833.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A. A. Zamyatnin, Jr., 2015, published in Uspekhi Biologicheskoi Khimii, 2015, Vol. 55, pp. 145-180.
Rights and permissions
About this article
Cite this article
Zamyatnin, A.A. Plant Proteases Involved in Regulated Cell Death. Biochemistry Moscow 80, 1701–1715 (2015). https://doi.org/10.1134/S0006297915130064
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297915130064