Abstract
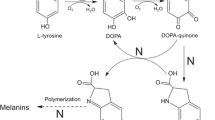
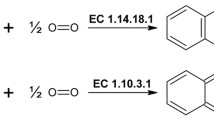
The recombinant tyrosinase from Verrucomicrobium spinosum bacteria has been obtained by heterologous expression in E. coli and isolated in the pure state to determine some of its biochemical properties (the kinetics of the enzymatic reaction, the effect of an inhibitor and activator, and the pH optimum). Coexpression of the genes for tyrosinase and recombinant adhesive mussel protein in one producer strain was used to introduce posttranslational modifications into the adhesive protein. These modifications involved the transformation of tyrosine residues into DOPA residues, which makes the protein capable of molecular adhesion in an aqueous medium.











Similar content being viewed by others
Notes
The online version of the article contains supplementary materials that are available free of charge on the journal website, http://www.biotechnology-journal.ru/.
REFERENCES
Haghbeen, K. and Tan, E.W., Direct spectrophotometric assay of monooxygenase and oxidase activities of mushroom tyrosinase in the presence of synthetic and natural substrates, Anal. Biochem., 2003, vol. 312, pp. 23–32. PMID: 12479831
Fairhead, M. and Thony-Meyer, L., Role of the C-terminal extension in a bacterial tyrosinase, FEBS J., 2010, vol. 277, no. 9, pp. 2083–2095. doi 10.1111/ j.1742-4658.2010.07621.x
Ensuncho, L., Alvarez-Cuenca, M., and Legge, R.L., Removal of aqueous phenol using immobilized enzymes in a bench scale and pilot scale three-phase fluidized bed reactor, Bioproc. Biosyst. Eng., 2005, vol. 27, pp. 185–191. doi 10.1007/s00449-005-0400-x
Ikehata, K. and Nicell, J.A., Characterization of tyrosinase for the treatment of aqueous phenols, Bioresour. Technol., 2000, vol. 74, pp. 191–199. PMID: 11925171
Tembe, S., Karve, M., and Inamdar, S., Development of electrochemical biosensor based on tyrosinase immobilized in composite biopolymeric film, Anal. Biochem., 2006, vol. 349, pp. 72–77. doi 10.1016/j.ab.2005.11.016
Nistor, C., Emneus, J., and Gorton, L., Improved stability and altered selectivity of tyrosinase based graphite electrodes for detection of phenolic compounds, Anal. Chim. Acta, 1999, vol. 387, pp. 309–326. doi 10.1016/ S0003-2670(99)00071-9
Irimia-Vladu, M., “Green” electronics: biodegradable and biocompatible materials and devices for sustainable future, Chem. Soc. Rev., 2014, vol. 43, no. 2, pp. 588–610. doi 10.1039/C3CS60235D
Koyanagi, T., Katayama, T., and Suzuki, H., Effective production of 3,4-dihydroxyphenyl-L-alanine (L-DOPA) with Erwinia herbicola cells carrying a mutant transcriptional regulator TyrR, J. Biotechnol., 2005, vol. 115, pp. 303–306. doi 10.1016/j.jbiotec.2004.08.016
Lee, B.P., Messersmith, P., and Israelachvili, J.N., Mussel-inspired adhesives and coatings, Annu. Rev. Mater. Res., 2011, vol. 41, pp. 99–132. doi 10.1146/ annurev-matsci-062910-100429
Lin, Q., Gourdon, D., Sun, C., and Holten-Andersen, N., Adhesion mechanisms of the mussel foot proteins mfp-1 and mfp-3, Proc. Natl. Acad. Sci. U. S. A., 2007, vol. 104, no. 10, pp. 3782–3786. doi 10.1073/ pnas.0607852104
Lu, Q., Danner, E., Waite, J.H., Israelachvili, J.N., et al., Adhesion of mussel foot proteins to different substrate surfaces, J. R. Soc. Interface, 2013, vol. 10, no. 79, pp. 56–68. doi 10.1098/rsif.2012.0759
Yu, J., Kan, Y., Rapp, M., et al., Adaptive hydrophobic and hydrophilic interactions of mussel foot proteins with organic thin film, Proc. Natl. Acad. Sci. U. S. A., 2013, vol. 110, no. 39, pp. 15 680–15 685. doi 10.1073/ pnas.1315015110
Clancy, S.K., Sodano, A., Cunningham, D.J., et al., Marine bioinspired underwater contact adhesion, Biomacromolecules, 2016, vol. 17, no. 5, pp. 1869–1874. doi 10.1021/acs.biomac.6b00300
Li, A., Mu, Y., Jiang, W., et al., A mussel-inspired adhesive with stronger bonding strength under underwater conditions than under dry conditions, Chem. Commun. (Camb.), 2015, vol. 51, no. 44, pp. 9117–9120. doi 10.1039/c5cc00101c
Jeon, E.Y., Hwang, B.H., Yang, Y.J., et al., Rapidly light-activated surgical protein glue inspired by mussel adhesion and insect structural crosslinking, Biomaterials, 2015, vol. 67, pp. 11–19. doi 10.1016/j.biomaterials.2015.07.014
Selinheimo, E., NiEidhin, D., and Steffensen, C., Comparison of the characteristics of fungal and plant tyrosinases, J. Biotechnol., 2007, vol. 130, no. 4, pp. 471–480. doi 10.1016/j.jbiotec.2007.05.018
Sevastyanov, O.V., Properties of tyrosinase of fungi Agaricus bisporus and its use for the elimination of chlorinated phenols, Bull. Odessa Natl. Univ. Chem., 2009, vol. 14, no. 3, pp. 28–35.
Kamal, U.Z., Ayesha, S.A., and Sharique, A.A., Purification and characterization of melanogenic enzyme tyrosinase from button mushroom, Enzyme Res., 2014, vol. 10, pp. 22–27. doi 10.1155/2014/120739
dos Santos, V.P.S., Mendonca, C., Pereira, P.R., et al., Compracao de metodos de extracao e caracterizacao da enzima tirosinase de agaricus bisporus, in XX Congresso Brasileiro de Engenharia Quimica, 2015, vol. 2, pp. 603–610. doi 10.5151/chemeng-cobeq2014-0435-25499-174494
Markova, E., Kotik, M., Krenkova, A., et al., Recombinant tyrosinase from Polyporus arcularius: overproduction in Escherichia coli, characterization, and use in a study of aurones as tyrosinase effectors, J. Agric. Food Chem., 2016, vol. 64, no. 14, pp. 2925–2931. doi 10.1021/acs.jafc.6b00286
Aoife, M.M., Evelyn, M.D., Sarah, B., et al., Biochemical characterisation of the coexisting tyrosinase and laccase in the soil bacterium Pseudomonas putida F6, Enzyme Microb. Technol., 2007, vol. 40, pp. 1435–1441. doi 10.1016/j.enzmictec.2006.10.020
Lim, S., Choi, Y.S., Kang, D.G., et al., The adhesive properties of coacervated recombinant hybrid mussel adhesive proteins, Biomaterials, 2010, vol. 31, no. 13, pp. 3715–3722. doi 10.1016/j.biomaterials.2010.01.063
Choi, Y.S., Yang, Y.J., and Yang, B., In vivo modification of tyrosine residues in recombinant mussel adhesive protein by tyrosinase co-expression in Escherichia coli, Microb. Cell. Fact., 2012, vol. 11, p. 139. doi 10.1186/1475-2859-11-139
Yang, B., Kang, D.G., Seo, J.H., et al., A comparative study on the bulk adhesive strength of the recombinant mussel adhesive protein fp-3, Biofouling, 2013, vol. 29, no. 5, pp. 483–490. doi 10.1080/08927014.2013.782541
Yu, J., Wei, W., Menyo, M.S., et al., Adhesion of mussel foot protein-3 to TiO2 surfaces: the effect of pH, Biomacromolecules, 2013, vol. 14, no. 4, pp. 1072–1027. doi 10.1021/bm301908y
Haemers, S., Koper, G.J., and Frens, G., Effect of oxidation rate on cross-linking of mussel adhesive proteins, Biomacromolecules, 2003, vol. 4, no. 3, pp. 632–640. doi 10.1021/ bm025707n
Monahan, J. and Wilker, J.J., Cross-linking the protein precursor of marine mussel adhesives: bulk measurements and reagents for curing, Langmuir, 2004, vol. 20, no. 9, pp. 3724–3729. PMID:15875406
Zeng, H., Hwang, D.S., Israelachvili, J.N., and Waite, J.H., Strong reversible Fe3+-mediated bridging between DOPA-containing protein films in water, Proc. Natl. Acad. Sci. U. S. A., 2010, vol. 107, pp. 12 850–12 853. doi 10.1073/pnas.1007416107
Hwang, D.S., Gim, Y., and Cha, H.J., Expression of functional recombinant mussel adhesive protein type 3A in Escherichia coli, Biotechnol. Prog., 2005, vol. 21, no. 3, pp. 965–970. doi 10.1021/bp050014e
Hwang, D.S., Yoo, H.J., Jun, J.H., et al., Expression of functional recombinant mussel adhesive protein Mgfp-5 in Escherichia coli, Appl. Environ. Microbiol., 2004, vol. 70, no. 6, pp. 3352–3359. doi 10.1128/ AEM.70.6.3352-3359.2004
Briceno, A., Munoz, P., Brito, P., et al., Aminochrome toxicity is mediated by inhibition of microtubules polymerization through the formation of adducts with tubulin, Neurotox. Res., 2016, vol. 29, pp. 381–393. doi 10.1007/s12640-015-9560-x
Zhou, J., Defante, A.P., Lin, F., et al., Adhesion properties of catechol-based biodegradable amino acid-based poly (ester urea) copolymers inspired from mussel proteins, Biomacromolecules, 2015, vol. 16, no. 1, pp. 266–274. doi 10.1021/bm501456g
Jing, Yu., Kan, Yajing., Rapp, M., et al., Adaptive hydrophobic and hydrophilic interactions of mussel foot proteins with organic thin films, Proc. Natl. Acad. Sci. U. S. A., 2013, vol. 110, no. 39, pp. 15 680–15 685. doi 10.1073/pnas.1315015110
Axambayeva, A.S., Shagyrova, Zh.S., Shustov, A.V., et al., Novel material: biocompatible glue for use in biology and medicine—recombinant mussel adhesive proteins, Eurasian J. Appl. Biotechnol., 2016, vol. 3, pp. 24–35.
ACKNOWLEDGMENTS
The work was supported by a grant from the Ministry of Education and Science of the Republic of Kazakhstan (no. 0115PK01798, “Bioadhesive Microparticles Releasing Antimicrobial Peptides, a New Preparation for Prophylaxis and Therapy of Caries”).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by Yu. Modestova
Abbreviations: DOPA—3,4-dihydroxyphenylalanine; CL—culture liquid; OD600—optical density at a wavelength of 600 nm; LB medium—Luria–Bertani medium; IMAC—immobilized metal affinity chromatography; IPTG—isopropyl-β-D-1-thiogalactopyranoside; NBT—nitro blue tetrazolium; PBS—phosphate buffered saline; SDS—sodium dodecyl sulfate; SDS-PAGE—polyacrylamide gel electrophoresis in the presence of SDS; and SLS—sodium lauroyl sarcosinate.
Rights and permissions
About this article
Cite this article
Aksambayeva, A.S., Zhaparova, L.R., Shagyrova, Z.S. et al. Recombinant Tyrosinase from Verrucomicrobium spinosum: Isolation, Characteristics, and Use for the Production of a Protein with Adhesive Properties. Appl Biochem Microbiol 54, 780–792 (2018). https://doi.org/10.1134/S0003683818080021
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683818080021