-
PDF
- Split View
-
Views
-
Cite
Cite
Tomomi Nakagawa, Masayoshi Kawaguchi, Shoot-applied MeJA Suppresses Root Nodulation in Lotus japonicus, Plant and Cell Physiology, Volume 47, Issue 1, January 2006, Pages 176–180, https://doi.org/10.1093/pcp/pci222
Close - Share Icon Share
Abstract
To maintain a symbiotic balance, leguminous plants have a systemic regulatory system called autoregulation of nodulation (AUT). Since AUT is schematically similar to systemic resistance found in plant–pathogen interactions, we examined the effects of methyl jasmonate (MeJA) or methyl salicylate (MeSA) on nodulation in Lotus japonicus. Shoot-applied MeJA strongly suppressed nodulation in the wild type and even hypernodulation in the har1 mutant, whereas MeSA exhibited no effect. MeJA inhibited early stages of nodulation, including infection thread formation and NIN gene expression, and also suppressed lateral root formation. These findings suggest that jasmonic acid and/or its related compounds participate in AUT signaling.
Plants survive in the environment amidst a vast amount of microorganisms, including pathogenic bacteria. To prevent invasions by harmful microorganisms, plants have evolved various defense systems involving pre-formed barriers and induced defense mechanisms. Recognition of invaders by plants triggers induced resistance which is locally activated at the infection site and also at uninfected tissues to protect the plant systemically against subsequent attack.
One well-studied phenomenon is systemic acquired resistance (SAR), which confers systemic resistance against a broad spectrum of plant pathogens and is characterized by an accumulation of salicylic acid (SA) and pathogenesis-related proteins (PRs) at the infection sites and in uninfected organs (for a review see Durrant and Dong 2004). The application of SA leads to the activation of SAR (for a review see Malamy and Klessig 1992). In contrast, expression of a bacterial salicylate hydrolase (nahG) gene, which inactivates SA by conversion to catechol, prevents the activation of SAR (Lawton et al. 1995). Therefore, SA is an indispensable and sufficient signal molecule for SAR induction.
Another kind of induced resistance is known as induced systemic resistance (ISR) (for a review see van Loon et al. 1998). A non-pathogenic bacterial strain, Pseudomonas fluorescens WCS417r, colonizing roots, has been shown to trigger ISR in the shoots of several plant species. Root colonization of WCS417r in Arabidopsis systemically prevents the proliferation of pathogenic bacteria such as Pseudomonas syringae pv. tomato, Fusarium oxysporum and Xanthomonas campestris pv. armoaciae (Pieterse et al. 2000). Plants unable to accumulate SA by introducing the nahG gene undergo normal WCS417r-induced ISR, whereas jasmonic acid (JA)- or ethylene (ET)-insensitive plant mutants fail to elicit ISR (Pieterse et al. 1996, Pieterse et al. 2002, Ton et al. 2002), indicating that JA or ET signaling is indispensable for ISR.
Such an ISR-like system is also documented in the symbiotic interaction between legumes and rhizobia (for a review see Caetano-Anolles and Gresshoff 1991). Kosslak and Bohlool (1984) demonstrated that plant resistance induced by rhizobial infection transmits from infected roots to uninfected roots and prevents further infection of rhizobia. This systemic regulation program in plants is called autoregulation of nodulation (AUT; Caetano-Anolles and Gresshoff 1991), and allows leguminous plants to keep the symbiotic balance by suppressing excessive bacterial invasion as well as nodulation that consumes a lot of photosynthates. To date, ET has been demonstrated to serve as a negative regulator of rhizobial infection. For example, an ET-insensitive mutant of Medicago truncatula, sickle, is hyperinfected by its symbionts, Sinorhizobium meliloti, but the nodulation zone in the sickle root is comparable with that in the wild type (Penmetsa and Cook 1997). In contrast, AUT-impaired mutants isolated from pea, soybean, Lotus japonicus and M. truncatula can be characterized by a hypernodulating phenotype with a wider nodulation zone (Caetano-Anolles and Gresshoff 1991, Szczyglowski et al. 1998, Wopereis et al. 2000, Kawaguchi et al. 2002, Penmetsa et al. 2003). Grafting experiments together with split root experiments using hypernodulating mutants indicate that AUT consists of two long-distance signals, a root-derived signal and an autoregulation signal. The former is generated in roots in response to rhizobia (especially in response to Nod factors), whereas the latter is produced in shoots, on receiving the root-derived signal. Recently, the first genes, HAR1 and NTS1 (NARK), that play a central role in AUT were identified by positional cloning in L. japonicus and soybean (Krusell et al. 2002, Nishimura et al. 2002, Searle et al. 2003); however, none of the other genes or endogenous signals related to AUT have been identified.
Like SAR and ISR, AUT is induced by bacterial infections and consequently exhibits systemic resistance against bacteria. In addition, it has been reported that L. japonicushar1 and soybean nts1 mutants were hyperinfected by a parasitic nematode, Meloidogyne incognita, as well as by arbuscular mycorrhizal fungi (Solaiman et al. 2000, Lohar and Bird 2003, Meixner et al. 2005). These findings led us to speculate that SA or JA that play a pivotal role in SAR or ISR may be involved in AUT in legumes. Based on the schematic similarity among these systemic resistances, we hypothesized that the mechanism(s) preventing excessive infection of bacteria and excessive nodulation in AUT may share some common components with SAR and ISR. To test this possibility, methyl salicylate (MeSA) or methyl jasmonate (MeJA) was applied to shoots that are considered to be a primary source of an unidentified autoregulation signal for nodulation (Caetano-Anolles and Gresshoff 1991).
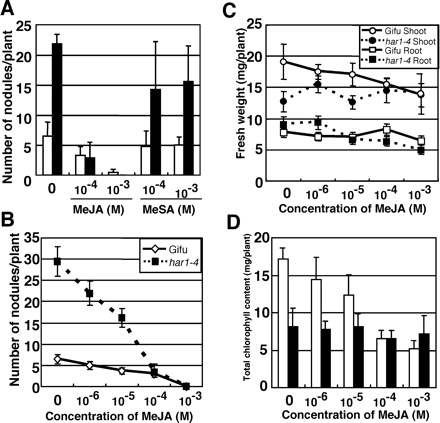
First, we examined the effects of MeSA and MeJA on nodulation using L. japonicus wild-type plants (Gifu B-129). As shown in Fig. 1A, MeSA at 10–4 and 10–3 M did not show a marked effect on nodulation. In contrast, shoot-sprayed MeJA at 10–4 and 10–3 M significantly suppressed nodulation (Fig. 1A). Notably, MeJA also strongly inhibited even the hypernodulating phenotype of the L. japonicushar1-4 mutant (Fig. 1A, 2), which is a strong allele of har1 with a missense mutation in the leucine-rich repeat (LRR) domain of a receptor-like kinase (Nishimura et al. 2002). A dose–response analysis indicated that both wild type and har1-4 had similar sensitivity to higher concentrations (10–4–10–3 M) of MeJA on nodulation; however, MeJA at lower concentrations (10–6–10–5 M) significantly inhibited the formation of nodules more in har1-4 than in wild type (Fig. 1B). Since the nodulation of wild-type plants is suppressed by AUT and har1-4 lacks the AUT signal, lower concentrations of MeJA could be more effective for nodule suppression in har1 mutants. Jasmonate and its related compounds are also known to inhibit plant growth, decompose photosynthetic pigments and promote senescence (Staswick et al. 1992). Nodule suppression by shoot-applied MeJA may be due to its secondary effect via plant growth inhibition. To address this question, we examined the effects of MeJA on plant growth and the Chl content. The application of MeJA (10–6–10–3 M) to the shoots of wild-type plants reduced both shoot and root fresh weights. In har1-4, MeJA had little inhibitory effect on plant growth at a range of 10–6–10–4 M (Fig. 1C). In contrast, the same concentrations of MeJA significantly inhibited nodulation in har1-4. Since the growth of har1-4 was shown to be strongly inhibited by hypernodulation, reduced nodulation might mask the growth defects produced by MeJA. We also measured the contents of total Chl after MeJA treatment. The application of MeJA to shoots of wild-type plants reduced the Chl content in a dose-dependent manner. MeJA ranging from 10–6 to 10–5 M had apparently no effect in har1-4 (Fig. 1D) but significantly suppressed nodulation. These results suggest that nodule suppression by MeJA may not be a secondary effect due to plant growth inhibition and pigment degradation.
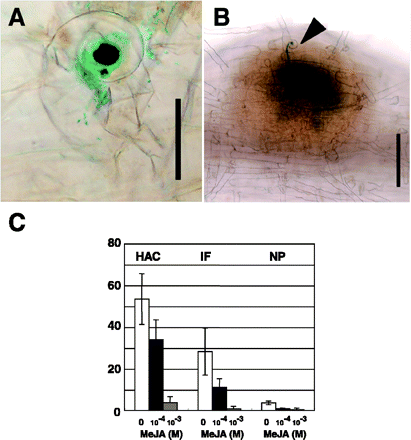
In order to define the stage of nodulation that was blocked by shoot-sprayed MeJA, wild-type seedlings were inoculated with M. loti NZP2235 carrying the lacZ gene. In wild-type plants without MeJA treatment, root hair curling with infection foci (bacterial colonization), infection threads and nodule primordia illuminated by blue staining were found 5 d after inoculation (Fig. 3A, B). When MeJA was applied at 10–4 M, root hair curling, infection threads and nodule primordia formation were significantly reduced (Fig. 3C). Notably, the application of MeJA at 10–3 M almost entirely blocked root hair deformation and curling. These results indicate that shoot-applied MeJA inhibits early stages of bacterial infection as well as nodule initiation.
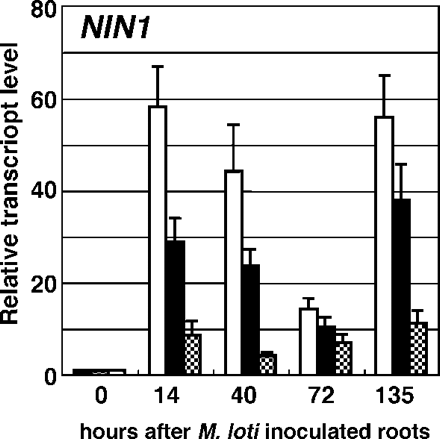
NIN encodes a putative transcriptional regulator required for infection thread formation and inception of the nodule primordia. NIN is also known as one of the early nodulin genes rapidly induced in response to Nod factors. The effects of MeJA application on the expression of NIN after M. loti inoculation were examined (Fig. 4). In our experimental conditions, NIN expression was highly induced at 14 h after M. loti inoculation in mock-treated plants. Application of MeJA at 10–4 M reduced the transcript level of NIN by 50% up to 40 h following inoculation, and at 10–3 M reduced the level by 15% in the same period. At 72 h after inoculation, the expression of NIN was transiently suppressed in the roots of mock-treated plants possibly by the action of AUT. The transcript level of NIN was fully recovered at 135 h after inoculation, whereas MeJA at 10–3 M still suppressed the NIN expression. These data suggest that the inhibition of infection and nodule formation by MeJA occurs upstream of the induction of NIN transcripts.
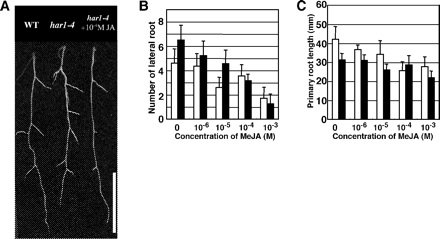
The lateral root is a post-embryonic organ that develops endogenously, like the root nodules. In the absence of rhizobia, har1 develops short primary roots with an increased number of lateral roots (Szczyglowski et al. 1998, Wopereis et al. 2000, Kawaguchi et al. 2002). Under our growth conditions, har1-4 plants formed slightly more lateral roots than the wild type 20 d after sowing (Fig. 5A, B). The exogenous addition of MeJA to the shoots reduced the number of lateral roots in a dose-dependent manner in both the wild-type and har1-4 plants. Depending on the increase in MeJA concentration, the growth of primary roots was also inhibited (Fig. 5C). The growth of the primary root in wild-type plants was more sensitive to MeJA than har1-4, whereas the formation of lateral roots in har1-4 was more sensitive to MeJA than wild-type plants. Interestingly, the application of MeJA at 10–4 M reduced the number of lateral roots by 50% whereas only a 10% reduction was found in the growth of primary roots of har1-4 plants. These results indicate that shoot-applied MeJA does not recover the short primary root phenotype of har1-4, but rather that increased lateral root formation is restored.
On the basis of schematic similarity among SAR, ISR and AUT signaling, we hypothesized that MeSA or MeJA may be involved in the systemic regulation of nodulation. Paying attention to a current model in which the AUT signal is produced in the shoots and then transmitted to the roots, we applied these substances to the aerial portion of plants. As a result, MeJA, but not MeSA, exhibited a strong inhibitory effect on nodulation. The effect could be observed even in the har1 hypernodulating mutant. In addition, MeJA also inhibited lateral root initiation. These findings indicate that HAR1 may possibly mediate the systemic regulation of nodule and lateral root development via activation of the production of endogenous jasmonates in the shoots. Since jasmonates have a vital role in ISR and systemic wound signaling to suppress subsequent invasion, it is possible that JA and/or its derivatives acts as an AUT signal.
One way to examine this hypothesis is to compare endogenous jasmonates between the wild type and the har1 mutant during the rhizobial infection process. It should be noted, however, that the analysis of endogenous JA and ET levels in the leaves of ISR-expressing plants revealed no changes in the production of these signal molecules (Pieterse et al. 2000). In addition, Verhagen et al. (2004) surveyed the transcriptional response of >8,000 Arabidopsis genes, none of which showed an altered expression pattern in the leaves of ISR-induced plants. Therefore, we consider that isolation of JA-deficient and -insensitive mutants and/or treatment of wild-type plants with a JA biosynthetic inhibitor that was developed recently would be a promising way to investigate involvement of JA signaling in nodulation and ISR studies. Finally, it should be noted that the effect of JA on nodulation contrasts with that on tuber formation in potato, which is positively regulated by JA derivatives such as tuberonic acid and its glucosides (Koda et al. 1988, Yoshihara et al. 1989).
Materials and Methods
In our all experiments, surface-sterilized seeds of L. japonicus B-129 Gifu and har1-4 were sown into sterile plastic growth boxes with sterilized vermiculite moistened with liquid B&D medium with or without 0.5 mM KNO3. All plants were grown in a Biotron LH-100 (Nihon Ika Co., Ltd, Osaka, Japan) under a 16 h light (100 µEm–1 S–2)/8 h dark cycle at 23°C. Rhizobia inoculation was performed as previously described (Nishimura et al. 2002). For treatments, 1 ml of MeJA (Wako, Osaka, Japan) or MeSA (Wako, Osaka, Japan) diluted in 10% ethanol was sprayed onto shoots at the indicated concentrations. Chl content was quantified by the method of Kirk and Allen (1965). Quantification of infection events was examined according to the method of Wopereis et al. (2000).
Total RNAs were isolated using an RNeasy Plant Mini Kit (Qiagen, Tokyo, Japan). Following DNase I treatment, reverse transcription was carried out with a QuantiTect Reverse Transcription Kit (Qiagen, Tokyo, Japan). Semi-quantitative PCR was performed on an ABI PRISM® 7000 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) using a QuantiTect™ SYBR® green PCR kit (Qiagen, Tokyo, Japan) to amplify the target transcripts from diluted cDNA. Sample volumes were normalized for equal amplification of DNA fragments with primers specific for the ATP synthase gene (AW719841). It was reported that ATP synthase has a constitutive expression profile (Radutoiu et al. 2003). PCR cycling conditions comprised an initial denaturation step at 95°C for 15 min followed by 40 cycles at 94°C for 15 s, 60°C for 30 s and 72°C for 45 s. The primers used for transcript amplification were: LjATPsyn-F, 5′-ACATGCTTGCACCATACCAA-3′; LjATPsyn-F, 5′-TCCCCAACTCCAGCAAATAC-3′; LjNIN1-F, 5′-CAATGCTCTTGATCAGGCTGTTGA-3′; LjNIN1-R, 5′-GAGTGCTAATGGCAAATTGTGTGTC-3′. Melting curve analysis was used to determine their identity.
Acknowledgments
We thank Yuko Ohashi and Shigemi Seo for helpful advice, and Hiroshi Kouchi and Asuka Kuwabara for advice and comments on the manuscript. This work was carried out with support from the Core Research for Evolutional Science and Technology (CREST) fund, Reserch Fellowships from the Japan Society for the Promotion of Science for Young Scientists, and the Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Fig. 1 Effect of MeJA on nodulation in Lotus japonicus. (A) Wild-type Gifu and har1-4 plants were inoculated with M. loti MAFF30–3099 and then sprayed with MeJA or MeSA on the aerial portion of seedlings 2 and 6 d after inoculation. (B) MeJA effect on nodulation. MeJA was sprayed 1 d before, and 1 and 6 d after inoculation. Fresh weight (C) and the Chl content (D) of plants shown in (B) were determined. Plants were harvested at 12 d after inoculation. Values shown represent the mean ± SD of at least 10 seedlings. Open bar, wild-type; solid bar, har1-4.
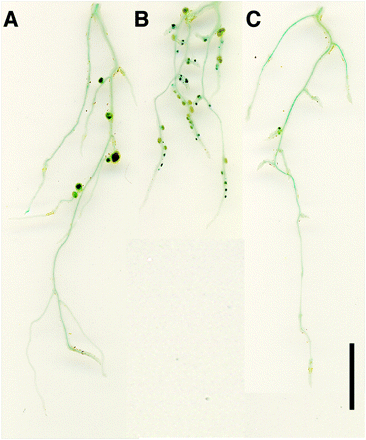
Fig. 2 MeJA suppressed the hypernodulation phenotype of har1-4 roots. Wild-type (A) and har1-4 (B and C) plants were inoculated with M. loti NZP2235 carrying the lacZ gene. The aerial portion of these plants was mock sprayed (A and B) or sprayed with 10–4 M MeJA 1, 5 and 8 d after inoculation. Plants were harvested 10 d after inoculation. Scale bar = 1 cm.
Fig. 3 MeJA effect on early nodulation response. Wild-type plants were inoculated with M. loti NZP2235 carrying the lacZ gene and, shortly after, aerial portions of seedlings were sprayed with MeJA. Plants were harvested 5 d after inoculation. The numbers of curled root hairs (A), infection threads (B, arrowhead) and nodule primordia (B) were counted (C). Scale bar = 50 µm in (A) and 200 µm in (B). Hac, root hair curling; IF, infection thread; NP, nodule primordia. Values shown represent the mean ± SD of at least 10 seedlings.
Fig. 4 Effect of MeJA on expression of NIN. Wild-type plants were mock sprayed (open bar), or sprayed with 10–4 M (filled bar) or 10–3 M (shaded bar) of MeJA 24 h prior to and 0 and 72 h after M. loti inoculation. Results are shown as fold increase compared with roots at time zero. Values shown represent the mean ± SD of three experiments.
Fig. 5 MeJA effect on lateral root formation under non-symbiotic conditions. Plants were grown in B&D medium containing 0.5 mM KNO3 for 20 d and then harvested. MeJA was sprayed 7, 12 and 17 d after germination. Values shown represent the mean ± SD of at least 10 seedlings. (A) Photograph of MeJA-treated roots. Scale bar = 1 cm. (B) Effect of MeJA on lateral root formation. (C) Effect of MeJA on primary root growth. Open bar, wild-type; filled bar, har1-4.
Abbreviations
- AUT
autoregulation of root nodulation
- ET
ethylene
- ISR
induced systemic resistance
- JA
jasmonic acid
- MeJA
methyl jasmonate
- MeSA
methyl salicylate
- SA
salicylic acid
- SAR
systemic acquired resistance
References
Caetano-Anolles, G. and Gresshoff, P.M. (
Kawaguchi, M., Imaizumi-anraku, H., Koiwa, H., Niwa, S., Ikuta, A., Syono, K. and Akao, S. (
Kirk, J.T. and Allen, R.L. (
Koda, Y., Omer, E.-S.A., Yoshihara, T. Shibata, H., Sakamura, S. and Okazawa, Y. (
Kosslak, R.M. and Bohlool, B.B. (
Krusell, L., Madsen, L.H., Sato, S., Aubert, G., Genua, A., et al. (
Lawton, K., Weymann, K., Friedrich, L., Vernooij, B., Uknes, S. and Ryals, J. (
Lohar, D.P. and Bird, D.M. (
Malamy, J. and Klessig, D.F. (
Meixner, C., Ludwig-Muller, J., Miersch, O., Gresshoff, P.M., Staehelin, C. and Vierheilig, H. (
Nishimura, R., Hayashi, M., Wu, G.-J., Kouchi, H., Imaizumi-Anraku, H., et al. (
Penmetsa, R.V. and Cook, D.R. (
Penmetsa, R.V. Frugoli, J.A., Smith, L.S., Long, S.L. and Cook, D.R. (
Pieterse, C.M.J., van Pelt, J.A., Ton, J., Parchmann, S., Mueller, M.J., Budhala, A.J., Métraux, J.P. and van Loon, L.C. (
Pieterse, C.M.J., van Wees, S.C.M., Hoffland, E., van Pelt, J.A. and van Loon, L.C. (
Pieterse, C.M.J., van Wees, S.C.M., Ton, J., van Pelt, J.A. and van Loon, L.C. (
Radutoiu, S., Madsen, L.H., Madsen, E.B., Felle, H.H., Umehara, Y., et al. (
Searle, I.R., Men, A.E., Laniya, T.S., Buzas, D.M., Iturbe-Ormaetxe, I., Carroll, B.J. and Gresshoff, P.M. (
Solaiman, M.Z., Senoo, K., Kawaguchi, M., Imaizumi-Anraku, H., Akao, S., Tanaka, A. and Obata, H. (
Staswick, P.E., Su, W. and Howell, S.H. (
Szczyglowski, K., Shaw, R.S., Wopereis, J., Copeland, S., Hamburger, D., Kasiborski, B., Dazzo, F.B. and de Bruijn, F.J. (
Ton, J., de Vos, M., Robben, C., Buchala, A.J., Métraux, J.-P., van Loon, L.C. and Pieterse, C.M.J. (
van Loon, L.C., Bakker, P.A. and Pieterse, C.M.J. (
Verhagen, B.W.M., Glazebrook, J., Zhu, T., Chang, H.-S., van Loon, L.C. and Pieterse, C.M.J. (
Wopereis, J., Pajuelo, E., Dazzo, F.B., Jiang, Q., Gresshoff, P.M., de Bruijn, F.J., Stougaard, J. and Szczyglowski, K. (