-
PDF
- Split View
-
Views
-
Cite
Cite
Mami Hosokawa, Shiro Suzuki, Toshiaki Umezawa, Yasushi Sato, Progress of Lignification Mediated by Intercellular Transportation of Monolignols During Tracheary Element Differentiation of Isolated Zinnia Mesophyll Cells, Plant and Cell Physiology, Volume 42, Issue 9, 15 September 2001, Pages 959–968, https://doi.org/10.1093/pcp/pce124
Close - Share Icon Share
Abstract
Tracheary element (TE) differentiation is a typical example of programmed cell death (PCD) in higher plants, and maturation of TEs is completed by degradation of all cell contents. However, lignification of TEs progresses even after PCD. We investigated how and whence monolignols are supplied to TEs which have undergone PCD during differentiation of isolated Zinnia mesophyll cells into TEs. Higher densities of cell culture induced greater lignification of TEs. Whereas the continuous exchanging of culture medium suppressed lignification of TEs, further addition of coniferyl alcohol into the exchanging medium reduced the suppression of lignification. Analysis of the culture medium by HPLC and GC-MS showed that coniferyl alcohol, coniferaldehyde, and sinapyl alcohol accumulated in TE inductive culture. The concentration of coniferyl alcohol peaked at the beginning of secondary wall thickening, decreased rapidly during secondary wall thickening, then increased again. These results indicated that lignification on TEs progresses by supply of monolignols from not only TEs themselves but also surrounding xylem parenchyma-like cells through medium in vitro.
(Received May 14, 2001; Accepted June 21, 2001).
Introduction
The vascular bundle in higher plants is a conductive system through roots and shoots. The vascular system is composed of two kinds of conducting tissues, the phloem for transportation of organic materials and the xylem for transportation of water and soil-derived nutrients. The vessels, the conducting tissue of the xylem, develop continuously (Aloni 1987). This continuous development of vessels appears to be led by intercellular interactions in vascular tissues in addition to polar auxin flow towards the roots (Sachs 2000).
The intercellular interactions during the development of vessels are complicated and difficult to study in vivo. The Zinnia experimental system established by Fukuda and Komamine (1980) in which single mesophyll cells differentiate into TEs, however, enabled investigations about the intercellular interactions during TE differentiation. In this system, at least two exogenous plant hormones, an auxin and a cytokinin, have been indicated to be necessary for induction of TE differentiation, and 30–50% of cells differentiate into TEs under proper hormonal condition (for review, see Fukuda and Komamine 1985). In addition to these hormones, several factors have been shown to be involved in intercellular interactions during TE differentiation. An oligosaccharide-like secreted factor in Zinnia conditioned medium appeared to play an important role in cell expansion and metaxylem-like TE differentiation (Roberts et al. 1997). Phytosulfokine-α secreted into Zinnia conditioned medium was suggested to be a factor responsible for TE differentiation (Matsubayashi et al. 1999). Xylogen, a kind of arabinogalactan protein, secreted into Zinnia conditioned medium was suggested to mediate local intercellular communication involved in TE differentiation (Motose et al. 2001). Higher pH induced cell expansion and delayed differentiation of metaxylem-like TEs (Roberts and Haigler 1994). These results indicate that various factors involved in intercellular interactions for TE differentiation were transferred between cultured Zinnia cells through the culture medium.
Lignins, complex phenolic heteropolymers, are deposited in the secondary cell walls in vivo, and function in mechanical support and solute conductance of higher plants. Lignin deposition also plays a role in disease protection (Whetten et al. 1998). Acquisition of the ability for lignin synthesis was probably associated with land colonization by plants (Boudet et al. 1995). Lignin synthesis has been investigated extensively in the Zinnia experimental system. Lignin is synthesized after the start of secondary cell wall thickening during TE differentiation (Fukuda and Komamine 1982). The activities or the levels of mRNA of major enzymes involved in lignin synthesis, for example phenylalanine ammonia-lyase (PAL), 4-coumarate:CoA ligase (4CL), cinnamyl alcohol dehydrogenase (CAD), and cell wall-bound peroxidases, were shown to be changed specifically in correspondence to TE differentiation (Fukuda 1997).
Furthermore, differentiation into TEs is a typical example of programmed cell death (PCD) in higher plants, and mature TEs are completed by the loss of all cell contents. The course of PCD during TE differentiation of isolated Zinnia mesophyll cells has been investigated extensively. The disruption of the central vacuole causes the release of nucleases (Thelen and Northcote 1989, Aoyagi et al. 1998) and proteases (Minami and Fukuda 1995, Ye and Varner 1996) that have been accumulated in the central vacuole. The release of these hydrolases would conduct the final degradation of all cell contents in TEs (Fukuda et al. 1998). Finally, perforation occurs on the primary wall at one of the longitudinal ends in single TEs (Nakashima et al. 2000). On the other hand, the TED4 protein, a tentative plant non-specific lipid transfer protein secreted into the apoplastic space, was suggested to act as an inhibitor of proteasome to protect other cells from undesirable injury due to proteolytic activities exudated from dying TEs (Endo et al. 2001).
The disruption of the central vacuole and successive breakdown of cytoplasmic streaming occur approximately 6 h after visualization of secondary cell wall thickenings in differentiating Zinnia cells (Groover et al. 1997). On the other hand, Kuriyama (1999) indicated the timing of cell death by following the viability of differentiating Zinnia cells during culture. He showed that all TEs were already dead cells at 72 h of culture, which was 8 h after the peak of the percentage of TE differentiation at 64 h of culture. Although these results showed that the irreversible step toward cell death, that is, the disruption of the central vacuole, would occur just after the completion of secondary cell wall thickenings, lignification of secondary cell walls progresses continuously until the completion of mature TEs (Fukuda and Komamine 1982). This may indicate that TEs that have undergone PCD are lignified by receiving monolignols from surrounding undifferentiated cells through the culture medium.
We analyzed the mechanism of lignification during TE differentiation of Zinnia mesophyll cells by changing the density of cell culture, by interrupting lignification with changes of culture medium or with an inhibitor of PAL, and by analyzing cultured medium with HPLC and gas chromatography-mass spectrometry (GC-MS). These studies showed that a novel mechanism of supplying monolignols to TEs mediated intercellular communication in xylem differentiation.
Results
Time course of TE formation and lignification
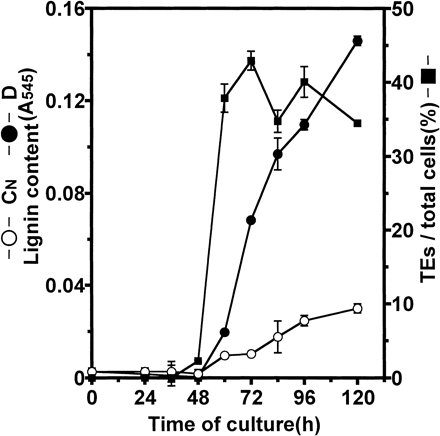
During culture of isolated Zinnia mesophyll cells in D medium, thickening of secondary walls started to be visible from 48 h of culture and the ratio of TEs to total cells reached 43% at 72 h of culture (Fig. 1). Accumulation of lignin in the cells cultured in D medium was first detected by phloroglucinol/acetyl bromide method at 60 h of culture, 12 h after the visibility of secondary cell wall thickening, and increased steadily until 120 h of culture. In CN culture, by contrast, neither TE differentiation nor an obvious increase in lignin content were detected.
Alteration of culture conditions
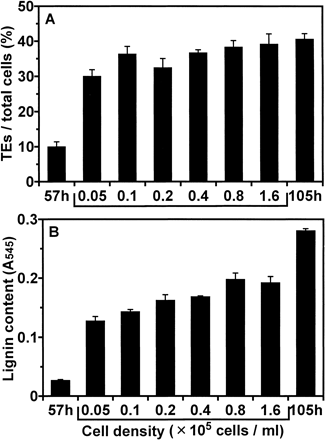
We hypothesized that higher cell culture density would result in heavier lignification by dint of the higher accumulation of lignin precursors secreted into the medium. Isolated mesophyll cells were cultured at the initial cell density of 0.8×105 cells ml–1 in D medium. After 57 h of culture, when thickening of secondary cell walls became visible, the cells were resuspended in fresh D medium at various cell densities (0.05, 0.1, 0.2, 0.4, 0.8, 1.6×105 cells ml–1) and cultured for additional 48 h. No significant inhibitory effect of cell dilution and exchange of medium on the ratio of TE differentiation was detected (Fig. 2A). On the other hand, the cells cultured at the higher cell density had the higher lignin content (Fig. 2B).
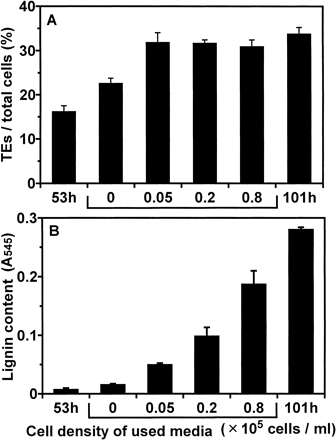
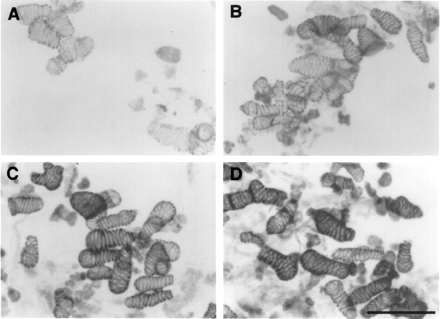
Furthermore, we investigated the effect of conditioned medium on lignification of TEs. After isolated mesophyll cells were cultured for 53 h in D medium, the cells were resuspended at low cell density (0.05×105 cells ml–1) in the conditioned media which had been used for cultures at each cell density (0.05, 0.2, and 0.8×105 cells ml–1) for 96 h, then cultured for further 48 h. As expected, lignin content was higher when conditioned medium of higher cell density was used (Fig. 3, 4).
Continuous exchanging of media during cell culture
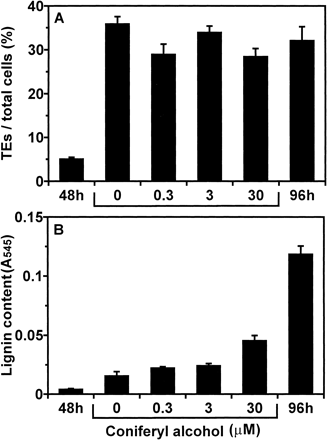
We expected that lignification should be suppressed by exchange of medium for removal of lignin precursors from cultured medium and addition of exogenous lignin precursors may overcome its inhibition. After isolated mesophyll cells were cultured for 48 h, the cells were collected and resuspended in fresh D medium or fresh D medium containing each concentration (0.3, 3, and 30 µM) of coniferyl alcohol (CA) at intervals of three hours for 48 h. After that, lignin content in the cells cultured under each condition was determined (Fig. 5). Lignification was almost perfectly inhibited by continuous exchanging of medium. On the other hand, treatment with higher concentrations of CA resulted in higher lignin content.
Inhibition of lignin deposition by AOPP and recovery by lignin precursors
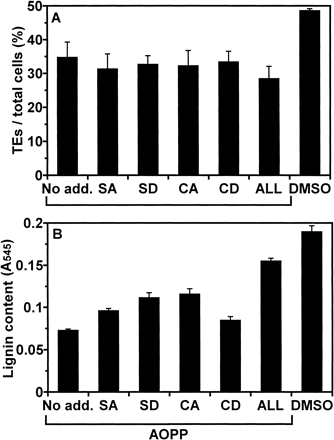
By addition of α-aminooxy-β-phenylpropionic acid (AOPP) at the start of culture, lignin content at 101 h of culture was decreased to less than half of that observed in the control culture (Fig. 6). However, by addition of CA, sinapyl alcohol (SA), and sinapaldehyde (SD) as lignin precursors at 30 µM, AOPP treated cells recovered lignification. The most effective lignin precursor was CA, then SA, and SD. Addition of all lignin precursors caused highest lignin deposition. This result verified that TEs can use lignin precursors in medium for lignification.
Analysis of lignin precursors in medium
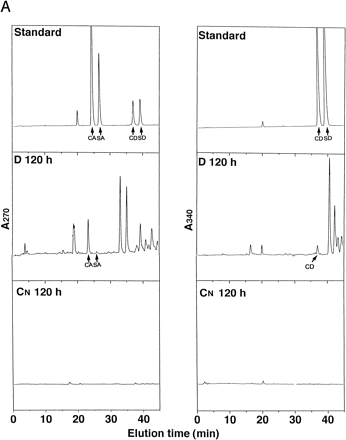
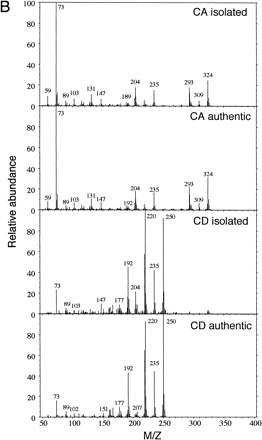
D and CN media after 120 h of culture were analyzed by HPLC (Fig. 7A). The peaks corresponding to CA and SA at 270 nm and coniferaldehyde (CD) at 340 nm were detected in D medium by co-elution with authentic compounds. However, none of these peaks were detected in CN medium. The medium of D culture was concentrated and fractionated by HPLC, and respective peaks of CA, CD, and SA were collected and analyzed by GC-MS. The MS results of GC peaks of CA and CD in D culture were identical to that of authentic CA and CD (Fig. 7B). Although mass spectrographs of SA showed that SA certainly exists in the fraction, the MS pattern had a little noise because of the low amounts analyzed (data not shown). Sinapaldehyde was not detected by HPLC and GC-MS.
Change in each lignin precursors in medium
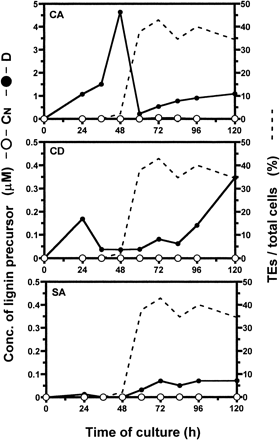
We followed the changes in concentration of three lignin precursors, CA, SA, and CD in medium during culture (Fig. 8). The concentration of CA was the highest of these three precursors. In D medium, CA concentration increased and peaked 4.6 µM at 48 h of culture when secondary wall thickening started. Between 48 h and 60 h of culture, CA concentration suddenly decreased to 0.2 µM. Thereafter, CA concentration increased steadily again. The concentrations of CD and SA remained at very low levels.
Discussion
Supply of monolignols to TE through medium
The appearance of TE was first observed at 48 h of culture. The rapid increase of the percentage of TE continued for 12 h, then ceased at 72 h of culture (Fig. 1). It was reported that the central vacuole was collapsed after approximately 6 h from visibility of secondary cell wall thickenings in differentiating Zinnia cells (Groover et al. 1997). In addition, it was indicated that all TEs were dead cells at 72 h of culture, that is 8 h after the peak of TE differentiation at 64 h of culture in the Zinnia experimental system (Kuriyama 1999). Based on these aspects, autolysis was estimated to be started in almost all TEs by 80 h of culture, that is 8 h after the peak of TE differentiation at 72 h of culture, in our experiments. The disruption of the central vacuole would cause the rapid cell death of TE by the release of various breakdown enzymes, such as Cys- and Ser-proteases (Minami and Fukuda 1995, Ye and Varner 1996), 43-kDa nuclease (Thelen and Northcote 1989), and two ribonucleases (Ye and Droste 1996). Nevertheless, the lignin content in D culture increased steadily from the first observation of secondary walls to 120 h of culture when autolysis was completely finished (Fig. 1). From this indirect evidence, it seemed that TEs use not only lignin precursors synthesized within each TE precursor cell, but also those transported from other cells through the culture medium, for lignin synthesis.
The experiments of changing culture conditions confirmed this hypothesis. The decrease of cell density of culture caused a decrease in the lignin content of resulting TEs (Fig. 2). If each TE precursor cell used only monolignols synthesized by itself and transported to its own secondary walls, a decrease of cell density of culture would not affect the lignin content of secondary walls of TEs. When the cells were cultured at low cell density in the conditioned media which had been used for cultures at each cell density, lignin content was higher in the cells grown in conditioned medium from cultures of higher cell density (Fig. 3, 4). These results were also consistent with the hypothesis. Moreover, continuous exchanging of media during cultures greatly suppressed final lignin contents in TEs (Fig. 5). This result indicated that TEs obtain considerable lignin precursors from outside through the culture medium. Furthermore, addition of lignin precursors to the culture in which lignification of TEs was inhibited by continuous exchanging of medium or treatment using AOPP overcame its inhibition (Fig. 5, 6). These results confirmed that TEs can use lignin precursors from the medium. The above results indicated that TEs which have undergone PCD would continue synthesizing lignin using monolignols supplied from non-differentiated cells through the medium.
Dynamic change of the concentration of lignin precursors in the medium
The ability of TEs to use CA, SA, and SD for lignification were shown by the experiment using AOPP (Fig. 6). On the other hand, CA was most abundant among these lignin precursors in the medium (Fig. 8). This result suggests that the lignin of TEs in D culture would be guaiacyl type and contain CA as a monolignol. This presumption is consistent with a previous report that, in the secondary xylem of Betula, the lignin of the vessels is mainly guaiacyl type, whereas that present in the fibres is guaiacyl-syringyl type and contains both CA and SA (Fergus and Goring 1970).
The concentration of CA in the medium peaked at 48 h of culture and decreased drastically by 60 h of culture during secondary cell wall thickening of TEs (Fig. 8). On the other hand, lignin contents in D culture increased steadily from 48 h to 120 h of culture (Fig. 1). Disagreement of a change of CA concentration in the medium and lignin content in D culture can be explained as follows. Before the start of secondary wall thickening of TEs, CA is simply accumulated into the medium by secretion from all or some cultured cells. The rapid decrease of CA concentration is caused by the start of CA incorporation into TEs and the reduction of CA supply from TEs undergoing PCD. Even after PCD of TEs, undifferentiated cells continue supplying CA for lignification of dead TEs. The secreted monolignols would be polymerized into lignin by wall bound peroxidases and/or laccases in seconday walls of TEs. It has been shown that TEs have peroxidase activities specific for TE differentiation in Zinnia experimental system (Sato et al. 1993, Sato et al. 1995). Laccase-like phenol oxidase activities have also been detected in Zinnia stems (Liu et al. 1994).
Coniferin, a phenolic glucoside of CA, is known to be produced and stored in the cambial sap in gymnosperms. However, in angiosperms limited species had been reported to produce and store monolignol-β-glucosides (Terazawa et al. 1984). Analysis about the possibility of participation of monolignol-β-glucosides in lignification during TE differentiation of Zinnia mesophyll cells is a future subject to be studied.
The comparison of the mechanism for transportation of lignin precursors to TEs between in vitro and in vivo
In the Zinnia experimental system, non-differentiated cells were suggested to be differentiated to xylem parenchyma-like cells through analyses of TED genes which are expressed in association with TE differentiation (Demura and Fukuda 1994). On the other hand, it had been shown that the activities of lignin-related enzymes, namely PAL (Fukuda and Komamine 1982), cinnamate 4-hydroxylase (C4H) (Ye 1996), and CAD (Sato et al. 1997) had still kept high levels in TE-inductive culture in Zinnia experimental system even after PCD of TEs. These results support that non-differentiated cells have the ability to produce monolignols. In addition, in transgenic poplar plants that harbored Eucalyptus gunnii CAD promoter and β-glucuronidase (GUS) gene fusion (Feuillet et al. 1995), GUS activity was detected mainly in the parenchyma cells located between TEs. Similarly, in transgenic tobacco plants that harbored PAL promoter-GUS or 4CL promoter-GUS gene fusion, GUS activities appeared not only in immature vessel but also xylem parenchyma cells (Bevan et al. 1989, Hauffe et al. 1991). In French beans, PAL and C4H were shown to accumulate in xylem parenchyma cells adjacent to metaxylem by immunogold techniques and visualisation by both light and electron microscopy (Smith et al. 1994). Tracheary element differentiation of cultured Zinnia mesophyll cells was suggested to be similar to primary xylem differentiation (Demura and Fukuda 1994, Roberts et al. 1997). Considering the results obtained in vitro and the expression and localization patterns of lignin-related enzymes in vivo, xylem parenchyma cells are thought to supply monolignols directly to adjacent vessels for their lignification through adjoining cell walls as apoplast in primary xylem of plants. However, there are many other lignified cells such as xylem fibers or phloem fibers in plant and the mechanisms of their lignification would be diverse (Wardrop 1981). Therefore, the knowledge obtained here would not simply apply to those lignified cells.
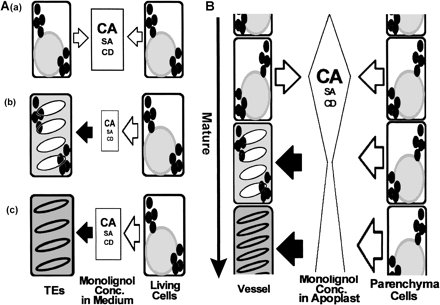
We summarized the results obtained here in an illustration (Fig. 9A). Before secondary wall thickening of TEs, monolignols may be secreted from all or some cultured cells (a). After start of secondary cell wall thickening of TEs, monolignols are incorporated by TEs while they are secreted from immature TEs and/or xylem parenchyma-like cells (b). After PCD of TEs, monolignols secreted from xylem parenchyma-like cells are incorporated by TEs (c). On the other hand, monolignols would be supplied from xylem parenchyma cells to adjacent vessels directly through adjoining cell walls as apoplast in primary xylem in vivo. (Fig. 9B). Further analysis of intercellular transportation of monolignols in vitro and in vivo would bring us to a better understanding of lignin biosynthesis.
Materials and Methods
Plant material and start of cell culture
Mesophyll cells were isolated from the first true leaves of 14-day-old seedlings of Zinnia elegans L. cv. Canary Bird (Takii Shubyo Co., Kyoto, Japan) as described previously (Fukuda and Komamine 1980, Fukuda and Komamine 1982). Isolated cells were cultured in the dark at 27°C with rotation on a revolving drum at 12 rpm in the following media: D medium, which contained 0.1 mg liter–1 1-naphthaleneacetic acid (NAA) and 0.2 mg liter–1 benzyladenine (BA) and which induced the differentiation of the cells into TEs; and CN medium, which contained 0.1 mg liter–1 NAA and did not support differentiation (Sugiyama et al. 1986).
Alteration of cell density during culture
Isolated mesophyll cells were cultured at the initial density of 0.8×105 cells ml–1 in D medium. After 48–60 h of culture, when thickening of the secondary wall became visible in the cultured cells, they were collected and washed. Then, equal numbers of cells (3.2×106 cells) were resuspended in fresh D medium at each of six cell densities (0.05, 0.1, 0.2, 0.4, 0.8, and 1.6×105 cells ml–1). Subsequently, cells were cultured for an additional 48 h and harvested by centrifugation (100×g, 1 min) or filtration (10 µm of nylon mesh) and used for measurement of lignin content, and respective culture media were filtered with 0.22 µm filter (Millex-GP; Millipore) and stored at –75°C.
Cell cultures in conditioned media
Isolated mesophyll cells were cultured until thickening of secondary cell wall became visible in the cultured cells as described above. Then, they were collected and washed. Equal numbers of cells (3.2×106 cells) were resuspended at the density of 0.05×105 cells ml–1 in conditioned media which had been used for culture at each cell density (0.05, 0.2, and 0.8×105 cells ml–1) for 96 h, filtered with 0.22 µm filter (Millex-GP; Millipore), and stored at –75°C as described above. Subsequently, cells were cultured for an additional 48 h and harvested by centrifugation (100×g, 1 min) or filtration (10 µm of nylon mesh) and used for measurement of lignin content.
Continuous exchanging of media during cell culture
After isolated mesophyll cells were cultured until thickening of secondary cell wall became visible in the cultured cells as described above, they were collected, washed, resuspended, and cultured in the same volume of fresh D medium at intervals of every three hours for a period of 48 h. The media used for resuspending the cells contained 0, 0.3, 3, or 30 µM of CA (Aldrich, Milwaukee, WI) by means of addition of thousand-fold concentrated stock solutions in dimethylsulfoxide (DMSO). After that, cells were used for measurement of lignin content.
Application of chemicals
Stock solutions of AOPP, CA, SA (Aldrich), CD (Aldrich), and SD (Wako Pure Chemical Industry, Osaka, Japan) were prepared in DMSO at 1,000-fold the required concentration. The inhibitor of PAL, AOPP, was added at 10 µM at the initiation of culture. Solution of CA, SA, CD, and SD were added after 53 h of culture. For control cultures, 0.01% DMSO was added.
Measurement of lignin content
For measurement of lignin content, cells (approximately 3.2×106 cells) cultured for various periods were ultrasonically homogenized (UD-200; TOMY, Tokyo) in 95% ethanol. After centrifugation at 1,000×g for 5 min, the pellet was washed three times with 95% ethanol and twice with ethanol-hexane (1 : 2, v/v). The washed pellet was allowed to air-dry. The lignin content of the samples was determined according to the method of Garcia and Latge (1987) with some modifications. The dried samples were ultrasonically resuspended (UD-200) in 90% ethanol, divided into four micro-tubes and centrifuged at 1,000×g for 5 min, and the ethanol was removed. To one tube 0.5 ml of 90% ethanol was added (blank) and to the other three 0.5 ml of 2% phloroglucinol in 90% ethanol was added (treatment). Five minutes later, 0.5 ml of 5.6 M HCl was added to each tube and mixed for 5 s. After 13 min incubation, tubes were centrifuged at 1,000×g for 5 min, the supernatants were removed, and the pellets were washed in 1 ml of 90% ethanol. After removing the ethanol, 0.5 ml of 25% acetyl bromide in glacial acetic acid was added to each tube, and the samples were shaken by hand for 5 s. Glacial acetic acid (0.5 ml) was then added and the tubes were mixed at 10 min intervals. Twenty-five min later, samples were centrifuged at 10,000×g for 5 min and the absorbance of the supernatant was measured at 545 nm to determine the lignin content.
Determination of lignin precursors in cultured media by HPLC
Thirty ml of cultured media were acidified by addition of 30 µl of acetic acid, and filtered through a 0.2 µm filter (PTFE; Millipore). Filtrates were applied to Sep-Pak C18 cartridges (Waters) that had been pre-wetted with 10 ml of ethanol and equilibrated with 10 ml of water containing 0.1% acetic acid. The cartridges were washed with 10 ml of water containing 0.1% acetic acid. The lignin precursors were eluted by 2 ml of a 60% ethanol solution.
Eluted samples were diluted with same volume of 2% (v/v) acetic acid and aliquots (20 µl) of them were fractionated by gradient HPLC on C-18 column (Lichrosorb PR18–5, 4.0×250 mm, GC Science Inc.) using solvent A: 2% (v/v) acetic acid; solvent B: 2% (v/v) acetic acid in acetonitrile; gradient conditions: 5–20% B over 45 min, 20–25% B over 5 min, 25–100% B over 4 min, 100–0% B over 1 min, 0% B for 5 min; solvent flow: 1 ml min–1. The eluate was monitored at 270 nm and 340 nm, and the peak areas of the eluting peaks were determined by integration.
GC-MS analysis
Two hundred and seventy ml of the medium of D culture for 96 h was concentrated and fractionated repeatedly by HPLC as described above, and respective peaks of CA, SA, and CD were collected. The collected samples were lyophilized and the resulting residues were extracted individually with methanol. Each methanol solution was dried in vacuo and subjected to GC-MS analysis.
Gas chromatography-mass spectrometry was performed on a JMS-DX303HF mass spectrometer (JEOL Ltd.) equipped with a Hewlett-Packard 5890J gas chromatograph and a JMA-DA5000 mass data system [electron impact mode, 70 eV; gas-chromatographic column, Shimadzu Hicap CBP-10M25–025 (5 m × 0.22 mm); temperature, 40°C at t=0 to 2 min, then to 240°C at 30°C min–1; carrier gas, He; splitless injection]. The samples for GC-MS were dissolved in N,O-bis(trimethylsilyl)acetamide and left standing at 60°C for 45 min; then an aliquot of the solution was subjected to GC-MS analysis.
All experiments were repeated several times to confirm the reproducibility of the results.
Acknowledgements
We are very grateful to Professor N. Amrhein of the Eidgenossische Technische Hochschule Zürich (Switzerland) for providing AOPP, the inhibitor of PAL. We thank Dr. Ross Whetten for critical reading of this manuscript, Dr. Hiroo Fukuda for critical review of this manuscript, and Dr. Masahiro Inouhe for helpful discussions and use of equipment. This work was supported in part by Grants-in-Aid from the Ministry of Education, Science and Culture of Japan (No. 09740599) and from the Japan Society for the Promotion of Science (JSPS-RFTF).
Corresponding author: E-mail, ysato@sci.ehime-u.ac.jp; Fax, +81-89-927-9630.
Fig. 1 Time course of TE formation and lignification during culture of isolated mesophyll cells of Zinnia. TEs were detected by observation of sculptured secondary wall thickenings under a light microscope in D culture (closed square). Lignin contents in D culture (closed circle) and CN culture (open circle) were determined as described in Materials and Methods. Vertical bars represent SD values for three replicates.
Fig. 2 Effect of alternation of cell density on lignification during culture. At 57 h of culture in D medium, cells were resuspended in fresh D medium at each cell density (0.05, 0.1, 0.2, 0.4, 0.8, and 1.6×105 cells ml–1). Cells were cultured for further 48 h (at 105 h of culture) and lignin contents were determined. The cells cultured for 57 h and 105 h of culture under the original conditions were designated as “57 h” and “105 h”, respectively. (A) The ratio of TE differentiation. (B) Lignin contents. Lignin contents were determined as described in Materials and Methods. Vertical bars represent SD values for three replicates.
Fig. 3 Effect of cultures in used media on lignification. At 53 h of culture, cells were resuspended at the density of 0.05×105 cells ml–1 in fresh medium or in conditioned media which had been used for culture at each cell density (0.05, 0.2 and 0.8×105 cells ml–1). Cells were cultured for further 48 h (at 101 h of culture) and lignin contents were determined. The cells cultured for 53 h and 101 h under the original conditions were designated as “53 h” and “101 h”, respectively. (A) The ratio of TE differentiation. (B) Lignin contents. Lignin contents were determined as described in Materials and Methods. Vertical bars represent SD values for three replicates.
Fig. 4 Light micrographs of phloroglucinol staining of cells cultured for 101 h in fresh medium (A) or in conditioned media which had been used for culture at each cell density (B, 0.05×105 cells ml–1, C, 0.2×105 cells ml–1, and D, 0.8×105 cells ml–1) as described in Fig. 3. Cells were stained with 1% phloroglucinol in 20% HCl. Bar: 100 µm.
Fig. 5 Effect of continuous exchanging of media on lignification during culture. After isolated mesophyll cells were cultured for 48 h, cells were resuspended in fresh D medium or fresh D medium containing each concentration (0.3, 3, and 30 µM) of CA at intervals of three hours for 48 h (at 96 h of culture) and determined lignin contents. For 0 µM of CA, DMSO was added. The cells cultured for 48 h and 96 h under the original conditions were designated as “48 h” and “96 h”, respectively. (A) The ratio of TE differentiation. (B) Lignin contents. Lignin contents were determined as described in Materials and Methods. Vertical bars represent SD values for three replicates.
Fig. 6 Effect of AOPP and lignin precursors on lignification during culture. At the initiation of culture, AOPP was added at 10 µM. At 53 h of culture, lignin precursors, CA, SA, CD, or SD, were added at 30 µM and “ALL” designates addition of all four lignin precursors at 30 µM each. For control, DMSO was added instead of AOPP or lignin precursors. Cells were cultured for further 48 h (at 101 h of culture) and lignin contents were determined. (A) the ratio of TE differentiation. (B) Lignin contents. Lignin contents were determined as described in Materials and Methods. Vertical bars represent SD values for three replicates.
Fig. 7 (A) Analyses of lignin precursors in cultured media by HPLC. Concentrated D and CN media at 120 h of culture were separated by reversed-phase HPLC on a C-18 column. Compounds eluting at 24.2, 26.5, 37.5, and 39.6 co-chromatographed with, in the sequence given, CA, SA, CD, and SD. The eluate was monitored at 270 nm and 340 nm. Standards containing 20 µM each lignin precursors in fresh D media were concentrated by the same method as samples. (B) Comparison of mass spectrum of authentic CA or CD and natural CA or CD in D culture after isolation by HPLC and gas chromatography.
Fig. 8 Changes in the concentration of each lignin precursor in medium during culture of isolated Zinnia mesophyll cells. Differentiation inductive (closed circle) and CN (open circle) media were separated by reversed-phase HPLC with monitoring at 270 nm and 340 nm.
Fig. 9 Model of lignification of TE undergoing PCD in vitro (A) and in vivo (B). (A) (a) Lignin precursors are secreted from all cells until initiation of secondary wall thickening, and lignin precursors are most accumulated in the medium at this stage. (b) During thickening of secondary walls of TE, cessation of secretion of lignin precursors from TE which have undergone PCD and initiation of incorporation of lignin precursors into secondary cell walls of TEs cause the rapid decrease of the concentration of lignin precursors in medium. (c) Increase of secretion of lignin precursors from parenchyma-like cells results in the steady increase of those concentrations in the medium. (B) A similar mechanism for lignification of vessels is expected to exist between vessels and adjacent xylem parenchyma cells mediated by adjoined cell walls as apoplast in primary xylem in vivo.
Abbreviations
- AOPP
α-aminooxy-β-phenylpropionic acid
- BA
benzyladenine
- CA
coniferyl alcohol
- CAD
cinnamyl alcohol dehydrogenase
- CD
coniferaldehyde
- C4H
cinnamate 4-hydroxylase
- 4CL
4-coumarate:CoA ligase
- DMSO
dimethylsulfoxide
- GC-MS
gas chromatography-mass spectrometry
- GUS
β-glucuronidase
- NAA
1-naphthaleneacetic acid
- PAL
phenylalanine ammonia-lyase
- PCD
programmed cell death
- SA
sinapyl alcohol
- SD
sinapaldehyde
- TE
tracheary element.
References
Aoyagi, S., Sugiyama, M. and Fukuda, H. (
Bevan, M., Shufflebottom, D., Edwards, K., Jefferson, R. and Schuch, W. (
Boudet, A.M., Lapierre, C. and Grima-Pettenati, J. (
Demura, T. and Fukuda, H. (
Endo, S., Demura, T. and Fukuda, H. (
Fergus, B.J. and Goring, D.A.I. (
Feuillet, C., Lauvergeat, V., Deswarte, C., Pilate, G., Boudet, A. and Grima-Pettenati, J. (
Fukuda, H. and Komamine, A. (
Fukuda, H. and Komamine, A. (
Fukuda, H. and Komamine, A. (
Fukuda, H., Watanabe, Y., Kuriyama, H., Aoyagi, S., Sugiyama, M., Yamamoto, R., Demura, T. and Minami, A. (
Garcia, S. and Latge, J.P. (
Groover, A., DeWitt, N., Heidel, A. and Jones, A. (
Hauffe, K.D., Paszkowski, U., Schulze-Lefert, P., Hahlbrock, K., Dangl, J.L. and Douglas, C.J. (
Kuriyama, H. (
Liu, L., Dean, J.F.D., Friedman, W.E. and Eriksson, K.-E.L. (
Matsubayashi, Y., Takagi, L., Omura, N., Morita, A. and Sakagami, Y. (
Minami, A. and Fukuda, H. (
Motose, H., Sugiyama, M. and Fukuda, H. (
Nakashima, J., Takabe, K., Fujita, M. and Fukuda, H. (
Roberts, A.W., Donovan, S.G. and Haigler, C.H. (
Roberts, A.W. and Haigler, C.H. (
Sachs, T. (
Sato, Y., Sugiyama, M., Gorecki, R., Fukuda, H. and Komamine, A. (
Sato, Y., Sugiyama, M., Komamine, A. and Fukuda, H. (
Sato, Y., Watanabe, T., Komamine, A., Hibino, T., Shibata, D., Sugiyama, M. and Fukuda, H. (
Smith, C.G., Rodgers, M.W., Zimmerlin, A., Ferdinando, D. and Bolwell, G.P. (
Sugiyama, M., Fukuda, H. and Komamine, A. (
Terazawa, M., Okuyama, H. and Miyake, M. (
Thelen, M.P. and Northcote, D.H. (
Wardrop, A.B. (
Whetten, R., MacKay, J.J. and Sederoff, R.R. (
Ye, Z.-H. (
Ye, Z.-H. and Droste, D.L. (