Abstract
The cancer treatments including chemotherapy, radiotherapy and other methods are effective in controlling cancer cells. However, their side effects are growing concerns to society. Application of nanotechnology in medicine may increase the effectiveness of the treatment. In this study, the silver nanoparticles were synthesised naturally from aqueous leaf extract of the Leucas aspera and their anticancer activity was investigated. Though, several studies were published on the effect of Leucas aspera nanoparticles on cancer cell lines, the in-depth study of anticancer activity of Leucas aspera nanoparticles was not investigated. Therefore, the present research work studied the characterisation and anticancer activity of Leucas aspera nanoparticles using HeLa cells as cancer cell model. The synthesis of silver nanoparticles was morphologically represented by change of colour from golden yellow to dark brown and also UV–visible spectral analysis provided a characteristic surface absorption peak at 450 nm. In addition to this, the size and surface charge of the nanoparticles were measured by zeta potential analyser, where the size was depicted to be 200 nm and zeta potential as −55.1 mV. Furthermore, Fourier transform infrared spectroscopy (FTIR) showed three intense peaks at 3343.705 cm−1, 1636.668 cm−1 and 668.336 cm−1 confirming the presence of flavonoids and polyphenols. Then, the SEM analysis with EDS showed the shape and size of the nanoparticles were round and 50 nm respectively. The cytotoxicity of the nanoparticles was determined by LDH assay, where 100 μg ml−1 of nanoparticles showed 50% of cytotoxicity and DNA fragmentation assay also denotes the death of the cells is through the induction of apoptosis. Furthermore, cell cycle analysis confirmed anticancer activity by showing 94.2% apoptotic cells during analysis. In summary, nanoparticles were proven to exhibit potent cytotoxic effect on cervical cancer cells and induce cell death through apoptosis. Since, the nanoparticles have exhibited cytotoxicity on the cancer cells; further investigation is required to understand their intracellular interaction.
Export citation and abstract BibTeX RIS
1. Introduction
Nanotechnology is an interdisciplinary branch of science where the methods of nanotechnology are applied in the field of biology. Nanotechnology has a wide range of applications in the field of medicine, food and health industries [1]. The peculiar feature of green synthesised nanoparticles attracted new field of research. Also due to the unprecedented structural, biological and optical properties they play a significant role in drug delivery [2]. It is also responsible for inhibition of abnormal cell growth and infectious agents [3]. The nanoparticles can be made from any sources like protein [4], metals etc, but the most commonly used sources are metal nanoparticles. Different metals including gold, silver, zinc, magnesium, titanium were used in the synthesis of nanoparticles [5]. Silver is a proven metal with antibacterial activity and other applications in the field [6]. The approach for synthesising nanoparticles is done by various physical and chemical methods [7] which are costly and unsafe to the environment. But, green synthesis of nanoparticle which involves plants is a cost effective and environmentally friendly alternative. Different parts of plant are used for the synthesis of nanoparticles [8]. The plant Leucas aspera is an important source for treating various acute illnesses. The hepatoprotective property of Leucas aspera was proven in rats by the inhibition of prostaglandins [9]. Also the plant has importance in traditional medicine as it is being used for chronic skin problems, cold, cough, and pain swelling [10]. The phytochemical analysis of plant has demonstrated the presence of flavonoids, alkaloids, tannins and triterpenoids [11], which are known to exhibit the anticancer properties. The plant is also known to have antioxidant, anti-inflammatory, antitumor, antiangiogenic properties with the ability to modulate the immune system [12]. The plant also demonstrated the anticancer activity in prostate cancer [13] and in lymphoma [14]. The employment of plant compounds is available in abundance and has fewer side effects with promising results in cancer treatment. Plant compounds are not stable in the body due to their less half-life [15] and also their inability to diffuse through the cells, such barriers are overcome by delivering through a vector/vehicle [16]. Silver nanoparticles being a prevalent type of nanoparticles with known properties are coupled with plant compounds for efficient delivery. Though the plant extract nanoparticles with methanol were reported in literatures [17] their degradation is expedited. The silver nanoparticles tendency to prevent the degradation of pharmaceutical compounds can be utilised as the efficient drug delivery system of plant compounds [18]. Nano drugs can easily diffuse into the cancer cells, compared to free drugs. Moreover, plant derived nano drugs provide increased benefits with less side effects on normal cells, increasing the bioavailability, stability and bioactivity of the drug [19]. Cancer is the fatal disease with high death rate in the past years and is predicted to increase in future [20]. Though many different methods are available for the treatment of cancer, not many could do their function without side effects and increased resistance of cancer. Therefore, the necessity for the search of new treatment could prevent or cure cancer. Cervical cancer is fourth most prevalent type of cancer in women. It originates from multiple factors, but 90% of the cases are caused by human papilloma virus (HPV) [21]. Current treatment for cervical cancer includes chemotherapy, radiation therapy and vaccines, which are overpriced and have less effect on cancer treatment. This current problem of modest treatment options has led to the development of new drugs and technologies to cure cancer. The current study aims to synthesise silver nanoparticles using aqueous extract of Leucas aspera as a reducing agent and stabiliser, which may act as a potential drug to treat cervical cancer cells. The preliminary cytotoxic studies of green synthesised nanoparticles were performed in in-vitro cell model for cervical cancer cells (HeLa cells) using LDH assay. Morphological changes of the treated cancer cells were observed through phase contrast microscopy. DNA fragmentation assay was performed to detect the apoptosis. The cell cycle analysis was performed using flow cytometry to investigate the inhibition of cell cycle stage.
2. Materials and methods
2.1. Chemicals and reagents
Dulbecco's modified eagle medium (DMEM), fetal bovine serum (FBS), and dimethyl sulfoxide (DMSO), antibiotics (penicillin and streptomycin), propidium iodide (PI), ethidium bromide (EtBr) were purchased from HiMedia Laboratories (Mumbai, India). Quercetin standard was purchased from Sigma-Aldrich (St. Louis, USA). Sodium pyruvate, NADH was purchased from SRL Chemicals (Mumbai, India).
2.2. Cell culture
The HeLa cell line was procured from Tamil Nadu veterinary and animal sciences university (Chennai, India). Cells were cultured in DMEM medium with 10% of fetal bovine serum and 1 ml of antibiotic solution, and then incubated at 37 °C in humidified CO2 incubator.
2.3. Sample collection
The Leucas aspera leaves were collected from SRM Institute of Science and Technology campus, Chennai and shade dried for a week for removing moisture from the sample. Then, it was subjected for grinding using mixer grinder. After sieving, uniform size of fine powder was obtained and stored in container for further studies.
2.4. Synthesis of nanoparticles
Synthesis of nanoparticles was performed using aqueous plant extract as described previously [22]. Briefly, 5 g of leaf powder was mixed with 100 ml of Millipore water. The solution was boiled for 10 min at 60 °C in the water bath and filtered using Whatmann filter. The filtrate collected was stored at 4 °C. Briefly, 45 ml of 1 mM silver nitrate solution was prepared, and 5 ml of aqueous leaf extract was added dropwise on constant stirring. The solution was then left on constant stirring to observe the colour change, which indicates the reduction of silver. The solution was centrifuged at 10,000 rpm for 10 min. The supernatant was discarded, and the pellet was stored for further use at 4 °C.
2.5. UV-Visible spectral analysis
The UV-Visible spectral analysis was performed for green synthesised nanoparticle according to [23]. Briefly 4 ml of the reaction mixture was taken in the quartz cuvette for spectral analysis. The spectroscopic readings were taken at different time intervals of incubation (30, 60, 120, 150 and 180 min). The spectral analysis was estimated using UV3600-Shimadzu UV-vis-NIR spectrophotometer with wavelength ranging from (300 nm–700 nm).
2.6. Dynamic light scattering and zeta potential
The green synthesise silver nanoparticles were investigated using zeta potential analyser to measure particle size and surface charge. The nanoparticle pellet was re-suspended in Millipore water and alalysed using Horiba Scientific nanoparticle analyser (SZ-100), Japan [24].
2.7. Scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS)
The green synthesised nanoparticles were subjected to scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS) to determine the morphological size, shape and elemental composition of synthesised nanoparticle [25]. The analysis was performed using the Ultra 55 Model-II Carl Zeiss SEM, Germany.
2.8. FTIR analysis
The green synthesised nanoparticle was investigated using FTIR analysis to determine the reactive functional groups involved in the interaction between the plant leaf extract and silver nitrate. The green synthesised nanoparticle pellet was re-suspended with 1 ml of deionised water and grounded with KBr pellet. Then, it was subjected to FTIR analysis using Agilent Technologies carry 660 FTIR spectrometer. The functional groups were identified using the reference peak values [26].
2.9. Lactate dehydrogenase assay (LDH assay)
Cells with a density of 0.5 × 105 were seeded in 24 well plates containing 1 ml of DMEM and incubated at 37 °C in humidified CO2 incubator for 24 h for cells to attach. After incubation, the cells were treated with three different concentrations of green synthesised nanoparticles (50 μg, 100 μg, 150 μg) and incubated for overnight. The untreated wells served as negative control whereas the quercetin treated cells served as positive control. After 24 h incubation, 50 μl media was transferred to 2 ml of LDH buffer and incubated in dark at room temperature for 30 min and substrate was added freshly before taking reading and absorbance was measured at 340 nm.
2.10. Phase contrast microscopy
Morphological changes of cervical cancer cells after green synthesised nanoparticles treatment were determined using phase contrast microscopy. The cell culture plate was treated with different concentrations (50, 100 and 150 μg ml−1) of green synthesised silver nanoparticles and incubated for 24 h in CO2 incubator. Then, the cell morphological changes were determined using phase contrast microscopy [27]. The images were captured using Olympus inverted microscope.
2.11. DNA fragmentation
The green synthesised silver nanoparticle treated cells (3 × 106) were trypsinised and suspended in 180 μl of lysis buffer (150 mM NaCl; 10 mM EDTA; 50 mM Tris.Hcl; 1% SDS) and incubated on ice for 20 min. After incubation 20 μl of RNase (20 mg ml−1) was added and incubated for 1 h at 37 °C. Further, 20 μl of proteinase K (20 mg ml−1) was added and incubated at 56 °C for 2 h. Then, DNA was separated using phenol-chloroform and isolated DNA was stored at −20 °C. The DNA samples were resolved in 1.5% agarose gel and visualised under gel documentation system (Biorad, USA) [28].
2.12. Cell cycle analysis
After green synthesised silver nanoparticles treatment, cells (1 × 106) were trypsinised and suspended in PBS and centrifuged at 1200 rpm for 5 min at room temperature. The supernatant was aspirated and pellet was re-suspended with 500 μl of PBS and washed again. The cells were fixed by adding 4.5 ml of ice cold ethanol and the tubes were incubated at −20 °C for one hour. Then, the suspension was centrifuged at 1500 rpm for 5 min and the supernatant was discarded. Cells were washed again with 5 ml PBS and centrifuged at 1500 rpm for 5 min. The supernatant was removed, and cells were suspended in 1 ml of propidium iodide (50 μg ml−1), and incubated in the dark for 30 min at room temperature [29]. Finally, the cells were analysed with BD FACS calibre flow cytometer (BD Biosciences, San Jose, CA) to determine apoptosis.
2.13. Statistical analysis
The statistical analysis of data obtained was performed using the software Graph Pad Prism 7.0 for Windows. All data obtained were represented as a mean ± standard error of the mean. One-way analysis of variance (ANOVA) was used for statistical analysis.
3. Result and discussion
3.1. Synthesis of nanoparticle
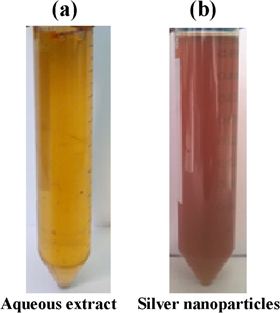
In the present study we demonstrated the effect of green synthesised silver nanoparticles on HeLa cells. The silver nanoparticles were reduced with the aqueous leaf extract of Leucas aspera. Previous studies have shown that the Leucas aspera exhibits immunomodulatory, anti-inflammatory and anti-oxidant properties and also reported that it inhibits the prognosis of cancer. It has also been reported in another study that it shows anticancer activity in lymphoma [14]. The organic chemicals present in the aqueous extract of Leucas aspera acts as a reducing and stabilising agent for synthesis of silver nanoparticles. The mixture of silver nitrate turned brownish from yellow after 45 min at 50 °C, which represents the reduction of silver to a nanoparticle as shown in figure 1.
Figure 1. Synthesis of silver nanoparticles/reduction of silver nitrate by aqueous leaf extract of Leucas aspera.
Download figure:
Standard image High-resolution image3.2. Characterisation of green synthesised silver nanoparticles
3.2.1. UV-Visible spectroscopy
After nanoparticles synthesis, the solution was analysed using UV-Visible spectroscopy to determine the shift in plasmonic resonance of silver. The shift was observed at absorbance peak of 450 nm as depicted in figure 2.The reduction of silver nitrate was clearly visualised in the spectral graph of UV-Visible spectroscopy. A shift in the absorbance peak was formed around 450 nm which infers the optimal reduction and formation of silver nanoparticles as reported in the previous study [23]. It was also reported that in the synthesis of silver particles by plant extracts, shift in the absorbance peak was formed at 450 nm [30] which, clearly demonstrates the nanoparticle synthesis. This shift in the absorbance peak is due to the plasmonic resonance of silver. The peak showing in this range confirms the synthesis and presence of AgNPs.
Figure 2. Shift in surface plasmonic resonance observed through UV-Visible spectroscopic analysis of silver nanoparticles synthesised from aqueous leaf extract of Leucas aspera.
Download figure:
Standard image High-resolution image3.2.2. Dynamic light scattering and zeta potential analysis
The green synthesised nanoparticles were further characterizsed by different techniques. Dynamic light scattering was performed to determine the size of the nanoparticles and zeta potential analyser was used to determine the surface charge of the nanoparticles. The mean diameter was found to be 200 nm as depicted in figure 3(a) and the zeta potential was measured as surface charge, which was around −55.1 mV as depicted in figure 3(b). The surface charge of the particle was −55.1 mV which is inferred to be good. If surface charge is more negative, it can promote optimal interactions with biomolecules. Similar type of result was obtained during the synthesis of AgNPs using Urtica dioca aqueous extract which infers the formation of silver nanoparticles [31]. Another study also reported that aqueous extract of Pedalium murex used for the synthesis of AgNPs has also shown similar type of results [32].
Figure 3. (a) The zeta potential (surface charge) of the silver nanoparticles synthesised from aqueous leaf extract of Leucas aspera is −55.1 mV. (b) The mean size of the silver nanoparticles synthesised from aqueous leaf extract of Leucas aspera analysed through dynamic light scattering (DLS) is 200 nm.
Download figure:
Standard image High-resolution image3.2.3. Scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS)
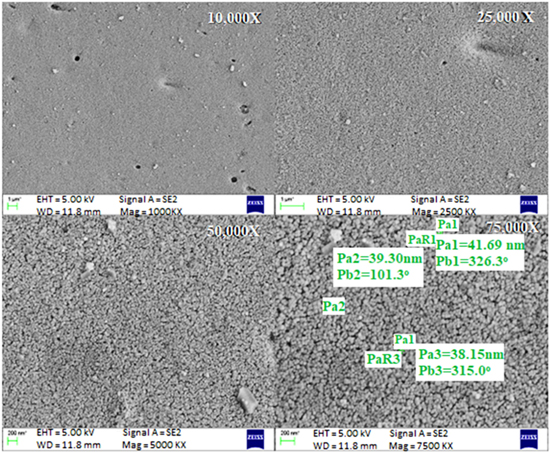
SEM is a powerful tool to analyse the surface and shape of the nanoparticles. Scanning electron microscopy is used to determine the particle size of the green synthesised silver nanoparticles, which was found to be 50 nm (figure 4). The particles under the range of 100 nm were considered nano and called nanoparticles [33]. The dynamic light scattering was used to determine shape of the green synthesised silver nanoparticles, which was found to be 200 nm which can be because of particle aggregates and formed complexes of nanoparticles. However, the result of scanning electron microscopy confirms the size of the nanoparticles. In addition the nanoparticle was also investigated with energy dispersive spectroscopy to determine the atomic elements in the nanoparticle as shown in figure 5. The silver was discovered in the spectra at 3 keV with an atomic percentage of 55.32 [5]. Previous studies have also reported that the nanoparticle size was around 25 nm–80 nm which is an optimal size for drug delivery [22] and energy peaks in the range of 2 KeV and 4 KeV [25].
Figure 4. Characterisation of silver nanoparticles synthesised from aqueous leaf extract of Leucas aspera using scanning electron microscopy (SEM).
Download figure:
Standard image High-resolution imageFigure 5. Indication of the presence of silver and its molecular composition in the nanoparticle synthesised from the aqueous leaf extract of Leucas aspera through EDS analysis.
Download figure:
Standard image High-resolution image3.2.4. FTIR analysis
FTIR spectroscopy is used to identify the presence of different functional groups that are present in the sample mixture by calculating the vibrational frequencies of the chemical bonds between the atoms [26]. Green synthesised silver nanoparticles were analysed by FTIR to determine the functional groups present in the synthesised nanoparticles. The elemental composition was interpreted using the software IRpal 2.0 and the results were similar to the previously reported studies as depicted in figure 6(a). Functional groups belonging to classes of carboxylic acids, alkynes etc, were identified. In present study FTIR analysis was applied to determine the functional group involved in the reduction of Ag+ to Ag0 and capping of biologically reduced silver nanoparticles synthesised using Leucas extract. The broad and wide peak existing at 3343 cm−1 as shown in figure 6(b) is specified for O–H functional group ensuring the presence of phenolic group and flavonoids [5]. The sharp broad absorption peak at 1636 cm−1 related to C=N indicating the presence of azomethines. They are considered under imines and aldimines. These results support that the compounds present in green synthesised AgNPs provide the stable interaction between plant and silver nanoparticles. The average intensity band seen in the region at 668 cm−1 represents C–H stretching of alkane region. Furthermore the band at wavenumber 549 cm−1 indicates the presence of C–Br functional group belongs to alkyl halides. Eventually, the result supports that presence of flavonoids/phenolic compounds acts as a reducing and stabilising agent.
Figure 6. (a) Illustration of reactive functional groups present in the aqueous plant extract through Fourier transform infrared spectroscopy (FTIR). (b) Infrared spectral peaks of several functional groups involved in formation of silver nanoparticle through aqueous extract of Leucas aspera. Spectral peaks were visualised at wave-numbers 3343.706 cm−1, 1636.668 cm−1 etc.
Download figure:
Standard image High-resolution image3.3. Lactate dehydrogenase assay (LDH assay)
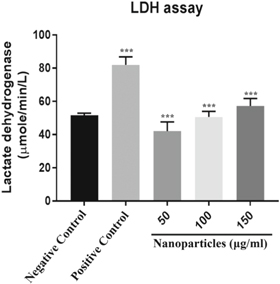
The green synthesised silver nanoparticles were treated to cancer cells and their cytotoxic effect was investigated. To determine the cytotoxicity of green synthesised nanoparticles on HeLa cells, cells were seeded in 24 well plates at a density of 50,000 cells per well and incubated for 24 h for the attachment. Cells were treated with different concentrations (50 μg, 100 μg, 150 μg) of nanoparticles and after 24 h incubation, LDH assay was performed. Lactate dehydrogenase is an enzyme which is present in animal cells that gets released when cells undergo damage or injury [34]. The LDH release from the damaged cells was measured to determine the cytotoxicity of the nanoparticles. The quercertin was used as a positive control. The obtained results showed that the increasing drug concentration increases the release of LDH from treated cells which infers to dose dependent toxicity shown in figure 7. The cytotoxicity (58%) was observed for the concentration of 150 μg ml−1. The increased release of LDH is the direct proportionality for the toxicity of cells. So, the concentration which has the highest LDH release is highly toxic [35]. A significant validation of LDH release was reported for AgNPs synthesised from Chrysanthemum indicum against 3T3 mouse embryo fibroblast cells [36].
Figure 7. Toxic nature of Leucas aspera silver nanoparticles on HeLa cell line is measured by lactate dehydrogenase assay. The values are compared with negative control as intended by a one way ANOVA and are entitled as mean ± SEM and were statistically significant (**** P < 0.001), n = 3 experiments were performed.
Download figure:
Standard image High-resolution image3.4. Phase contrast microscopy
A dose dependent morphological change in the cell culture plate treated with the green synthesised silver nanoparticles was observed through phase contrast microscopy. Dysplastic morphology with cell blebs was seen along with the increased cell death in correspondence to increase of concentration of nanoparticle treatment which is shown in figure 8. At lower concentrations, cells remained intact with less dead cell population, whereas at higher concentrations the number of dead cells increased along with the cell shredding, which indicates that the cell death is the result of apoptosis. The cells were incubated with drug for 24 h before they were visualised under microscope. Similar results were reported in LoVo cells when treated with AgNO3 nanoparticles synthesised by the reduction of plant extract from Ferula asafoetida [27].
Figure 8. Phase contrast microscopy images of Leucas aspera silver nanoparticle treated HeLa cells in the magnification of 20×.
Download figure:
Standard image High-resolution image3.5. DNA fragmentation
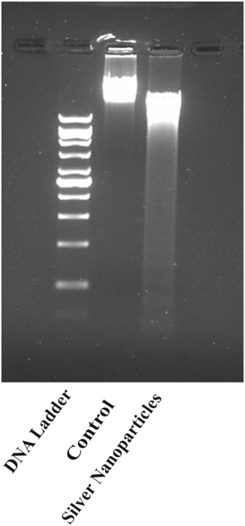
The cells were treated with green synthesised silver nanoparticles to determine the induction of DNA damage. The cells were treated with 150 μg ml−1 drug concentration and incubated for 24 h. The visualisation of isolated DNA in agarose gel shows the formation of DNA ladder as depicted in figure 9 which indicates the occurrence of apoptosis [37]. Apoptosis is the major signal to maintain homeostasis in the body [38]. Induction of apoptosis results in the cleavage of DNA. The physiological characteristics of apoptosis include the membrane flipping, DNA damage, apoptotic bodies [39]. In the present study the cells were treated with synthesised silver nanoparticles resulting in the induction of apoptosis. DNA isolated from untreated cells was used as a negative control, and the 1Kbp ladder was used as a marker. The results that were obtained showed that green synthesised nanoparticles induced the DNA damage at 150 μg ml−1 concentration. The reports have shown that the synthesised biogenic nanoparticles induce apoptosis more efficiently than control [38]. The DNA was fragmented at the given concentration that infers the induction of apoptosis. Similar kind of band patterns is visualised in the investigation of toxic effect of AgNPs synthesised from Solanum nigrum against hepatic cells [40].
Figure 9. DNA fragmentation in HeLa cells after treatment with synthesised silver nanoparticles. The isolated DNA was analysed through 1.5% agarose gel electrophoresis.
Download figure:
Standard image High-resolution image3.6. Cell cycle analysis
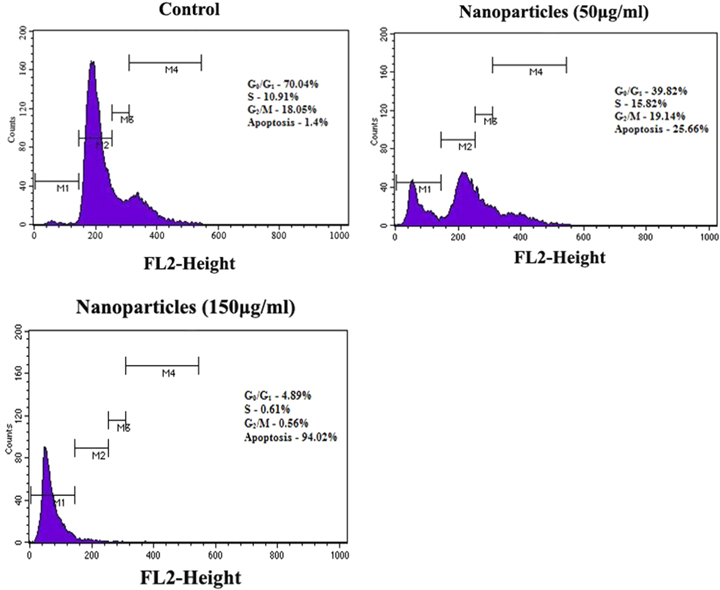
The agents that have ability to induce apoptosis also possess the ability to inhibit cell growth [36]. To determine effect of nanoparticles on cell growth, cell cycle analysis was performed using flow cytometry. Control cells and cells were treated with different concentrations (50 and 150 μg ml−1) of nanoparticles. After incubation, they were stained with propidium iodide. In this study, different phases of the cell cycle were analysed in treated cells and control cells. G1 and G2 phases are responsible for protein synthesis and S phase for DNA replication [27]. Obtained results showed that cells treated with nanoparticles induce apoptosis. Green synthesised silver nanoparticle completely arrested the cell cycle and results have suggested that the nanoparticles inhibited the cell growth at almost every phase of cell cycle, i.e., G0/G1, S, G2/M phases as shown in figure 10. Similar kind of results was observed in the investigation of cytotoxic effect of biosynthesised AgNPs using Nepeta deflersiana against the human cervical cancer cell lines (HeLa) [41].
Figure 10. Silver nanoparticles inhibit cell cycle at different stages in dose dependent manner which was assessed through flow cytometry.
Download figure:
Standard image High-resolution image4. Conclusion
In the present study we demonstrated that the green synthesised nanoparticles from Leucas aspera exhibit anticancer activity by inducing apoptosis in HeLa cancer cells. The aqueous leaf extract of Leucas aspera was used to synthesise the silver nanoparticles. The synthesised AgNPs has the potential to reduce the silver nitrate into silver, shown by colour change from golden yellow to dark brown. Further, UV spectral analysis was performed to characterise the morphological changes exhibits plasmonic absorption shift at 450 nm. The size of the nanoparticles was also analysed by SEM, which was 50 nm within the standard nanoparticle size. In addition, presence of the silver in the synthesised nanoparticles from aqueous leaf extract of Leucas aspera was confirmed with EDS. The FTIR investigation determines the phenolic compounds and flavonoids in green synthesised silver nanoparticles. The LDH assay showed that AgNPs increased cells toxicity in dose dependent manner and DNA fragmentation assay result showed that AgNPs induced apoptosis by fragmenting the DNA. Furthermore, cell cycle analysis results showed that cell cycle arrest was induced by green synthesiased silver nanoparticles. This demonstrates that, green synthesised silver nanoparticles may act as a potential chemotherapeutic agent to control cancer cells. Finally, our future aim is to investigate the effect of green synthesised nanoparticles on signalling molecular mechanism responsible for cancer cell inhibition.
Acknowledgments
We are grateful to Dean and HoD, Department of Biotechnology, School of Bioengineering for providing infrastructure to work. We take special privilege to thank our Director, Engineering & Technology and Management, SRM Institute of Science and Technology for funding chemicals for this project.