Abstract
Predictions of warmer droughts causing increasing forest mortality are becoming abundant, yet few studies have investigated the mechanisms of forest persistence. To examine the resistance of forests to warmer droughts, we used a five-year precipitation reduction (∼45% removal), heat (+4 °C above ambient) and combined drought and heat experiment in an isolated stand of mature Pinus edulis-Juniperus monosperma. Despite severe experimental drought and heating, no trees died, and we observed only minor evidence of hydraulic failure or carbon starvation. Two mechanisms promoting survival were supported. First, access to bedrock water, or 'hydraulic refugia' aided trees in their resistance to the experimental conditions. Second, the isolation of this stand amongst a landscape of dead trees precluded ingress by Ips confusus, frequently the ultimate biotic mortality agent of piñon. These combined abiotic and biotic landscape-scale processes can moderate the impacts of future droughts on tree mortality by enabling tree avoidance of hydraulic failure, carbon starvation, and exposure to attacking abiotic agents.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Introduction
Tree mortality due to warming and drought is an increasingly observed feature of global forests (Allen et al 2015). Tree mortality has more than doubled throughout much of the Americas in the last forty years (McDowell et al 2018), consistent with a global temperature-driven impact (e.g. Breshears et al 2005, Adams et al 2009, Williams et al 2013). Drought experiments that kill trees under warmer conditions result in faster death (Duan et al 2014, Allen et al 2015, Adams et al 2017a). The increased mortality under such hotter droughts is related to both temperature and vapor pressure deficit (VPD) impacts on carbon and water metabolism (Adams et al 2017b). Predictions suggest that the combination of drought extremes and climate warming, e.g. e.g. global-change-type droughts (Breshears et al 2005, 2009) or hotter droughts (Allen et al 2015) will increase tree mortality globally.
Piñon pine-juniper (Pinus edulis-Juniperus monosperma) woodlands have provided an useful system for examining the mechanisms of mortality and survival over the last decade (Breshears et al 2018), from theoretical (e.g. McDowell et al 2008), observational (e.g. Breshears et al 2005, 2009, Redmond et al 2015) and experimental standpoints (e.g. Plaut et al 2012). The experimental value of these woodland systems arises because (a) they are feasible to manipulation, with mature trees only 3–6 m tall, (b) pine and juniper are sympatric yet they have very different hydraulic and carbon metabolism strategies during droughts (e.g. West et al 2008, Limousin et al 2013, Dickman et al 2015), and (c) they have divergent rates of death during drought (Breshears et al 2005, Shaw et al 2005). Regional droughts and a previous field drought-manipulation both resulted in pine mortality within 12 months, followed by juniper mortality after ∼24 months, which were tied closely to prolonged periods of very negative pre-dawn water potential (Breshears et al 2009, Plaut et al 2012). Additionally, heat accelerates mortality in piñon pine saplings (Adams et al 2009) and seedlings (Adams et al 2017a). At the site described in this paper, however, experimental drought and heat manipulations failed to induce mortality even after five years of treatment (Adams et al 2015, Grossiord et al 2017a). Here, we address the mechanisms that underlie this surprising survival.
A critical challenge given the empirical and process-model predictions of increasing mortality rates in coniferous systems is understanding the potential mechanisms of tree persistence under drought and heat. Unfortunately, these mechanisms are rarely quantified, potentially resulting in overestimates of predictions of future forest loss (Keppel et al 2012, Lloret et al 2012, McDowell et al 2016). Topographic positions that enable access to quasi-permanent soil water sources are among the plausible mechanisms underlying plant survival to drought and heat, based on observational and modeling studies (Allen and Breshears 1998, Redmond et al 2015, McLaughlin et al 2017, Tai et al 2017). To understand the persistence of trees in the future we can also consider cause-and-effect field experiments in mature forests using treatments that simulate drought under warmer conditions. Such experiments can be applied to forests to investigate just how severe a hotter-drought they can survive, and the mechanisms by which they survive or die.
We conducted five-year experimental manipulations of precipitation (∼45% reduction, referred to herein as drought), temperature (+4 °C above ambient) and combined drought and increased temperature relative to a control in a mature piñon pine-juniper woodland to examine the mechanisms of mortality and survival under drought and elevated temperature. Multiple papers have been published from this experiment (Adams et al 2015, Grossiord et al 2017a, 2017b, 2017c, McBranch et al 2018) but none have focused on understanding the mechanism(s) that may have promoted the tree survival of drought and heat. Our initial hypothesis was that tree persistence under experimental drought and warming should be associated with lower evidence of hydraulic failure and carbon starvation, and greater belowground water uptake (e.g. McLaughlin et al 2017). Our empirical and modeling approaches allowed testing of this hypothesis from hydraulic and carbon-based perspectives in a comprehensive manner to allow strong inference. Additionally, we measured insect abundance and attack to investigate the role of biotic attack in persistence under warming and drought.
Methods
Experimental design
We measured the critical parameters associated with hypothesized mechanisms of mortality and survival (McDowell et al 2011, Martínez‐Vilalta et al 2014, Anderegg et al 2015, Johnson et al 2018), including species-specific pre-dawn water potential thresholds for mortality (McDowell et al 2016), iso/anisohydry (Martínez-Vilalta et al 2014), branch percent-loss-conductivity (PLC, Anderegg et al 2015), whole-plant-PLC (McDowell et al 2013), whole-tree leaf area:sapwood area ratio (Al:As; Mencuccini 2003), water source depths (Grossiord et al 2017a), hydraulic conductance and loss thereof (Sperry and Love 2015), whole-plant non-structural carbohydrates (NSC; Zhao et al 2013), insect abundance and insect attack rates (Gaylord et al 2013), and normalized difference vegetation index (NDVI) and % crown brownness (Gaylord et al 2013) as indexes of canopy impacts. We then utilized the site-calibrated ecohydrological model TREES (Mackay et al 2015) to provide further interpretation regarding the mechanisms underlying pine and juniper responses to drought and heat within the stand. The combination of these measurement and model variables allows a comprehensive test of the potential mechanisms that underlie survival of trees under drought and heat.
Site description
The study was conducted at the Los Alamos Survival-Mortality (SUMO) experiment located in Los Alamos County, New Mexico (35.49°N, 106.18°W, 2175 m a.s.l). The site is located on a mesa-top and is characterized by Hackroy clay loam soils derived from volcanic tuff (Soil Survey Staff, Natural Resources Conservation Service, United States Department of Agriculture, http://websoilsurvey.nrcs.usda.gov) with a soil depth ranging from 40–55 cm based on monthly soil-coring done for soil water isotope collection (Grossiord et al 2017a). The vegetation is dominated by piñon pine (Pinus edulis Engelm.) and one-seed juniper (Juniperus monosperma (Engelm.) Sarg.). Grasses, cacti and other tree species such as Gambel oak (Quercus gambelli Nutt.) can be found in inter-canopy spaces but they do not contribute significantly to total stand biomass. The experimental site covers an area of approximately 1.0 ha. The site is surrounded by an extensive area of pine trees that died in 2002–2003 during a severe regional drought (Breshears et al 2005, 2009, Garrity et al 2013). Outside of the 1.0 ha experimental area, there are no live, mature piñon pine trees within at least 1 km, based on surveys. This refugium was selected from four potential local refugiums (isolated patches of woodland amidst a landscape of dead trees) because it was the only one located on a mesa (the other three were located in valleys), and because it had the most positive foliar carbon isotope ratios of all the sites (∼−22‰ versus −24‰ at the other three sites), indicative of the greatest water stress. The climate is semi-arid, with a mean annual temperature of 10.1 °C and a mean annual precipitation of 360 mm (1987–2016 mean), with about 50% falling during the North American Monsoon season from July to September (http://environweb.lanl.gov/weathermachine).
At the start of the growing season in 2012, we installed open-top chambers increasing air temperature by ∼4.0 °C and a precipitation exclusion structure consisting of clear polymer troughs reducing incoming precipitation reaching the ground by 45%. 64 trees were randomly selected for the experiment (32 juniper and 32 piñon pine trees, >3 cm diameter at breast height). Mean tree age was 56 ± 5 years and 79 ± 7 years for piñon and juniper, respectively (determined from tree cores). Tree height ranged between 1.5–4.5 m. Chamber tops exceeded tree-tops by at least 1 m. The trees were assigned to five treatments (5–6 trees per treatment and per species): ambient for trees in ambient temperature and precipitation, control chamber for trees within chambers set to maintain ambient temperature and precipitation, warming for trees inside chambers where temperature was maintained at ∼4.0 °C above ambient temperature, drought for trees located within the precipitation exclusion structure, and drought and heat for trees where both treatments were applied simultaneously (figure S1 is available online at stacks.iop.org/ERL/14/045014/mmedia).
Chamber footprints ranged from 6–20 m2 and contained between one and five trees located at a minimum distance of 1.5 m from the chamber boundary. The selected trees in the drought treatment were located at least 10 m from the border of the precipitation exclusion structure (equivalent to two times the height of the tallest tree in the drought treatment). No soil barriers were installed to avoid root damage. Climatic conditions were measured continuously and recorded by two weather stations at the site (Climatronics, Bohemia, NY, USA). Atmospheric temperature and relative humidity were measured in all chambers using C215 Campbell sensors (Campbell Scientific, Logan, UT, USA) at two positions (1 m height and 2/3 of the canopy) and used for controlling the industrial-scale air-conditioning units that regulated chamber temperature. Further site details can be found in (Grossiord et al 2017b).
Relative extractable water
Soil drought intensity experienced by trees was estimated using the daily relative extractable water in the soil over the whole root zone (REW, unitless) per Grossiord et al (2017a). This value varies between 1, i.e. field capacity, and 0, i.e. permanent wilting point. We used the forest water balance model BILJOU to estimate REW at a daily-time scale by using measurements of daily climatic conditions at the site (rainfall, radiation, windspeed, and air temperature and humidity). At a daily-time scale, this model calculates the different water fluxes in the ecosystem: tree transpiration, understory evapotranspiration, rainfall interception and drainage, which are all dependent on leaf area index (LAI, m2 m−2) and evaporative demand (i.e. the potential evapotranspiration, mm). REW at this site correlates well with both measured soil water content (top 15 cm) and plant pre-dawn water potentials (Grossiord et al 2017a). For example, we observed a strong relationship between REW and mean soil water content over the 0–50 cm soil profile (y = 23.95x +6.02; R2 = 0.68; P < 0.001) demonstrating that simulations of REW from the model reflected the soil water content (above the bedrock) at our site (Grossiord et al 2017a).
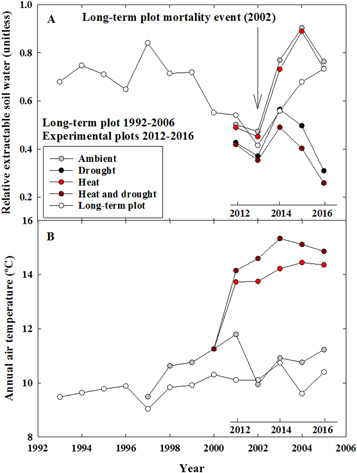
We measured LAI with a LAI-2000 Plant Canopy Analyzer (PCA, LI-COR, Lincoln, NE, USA) in June 2015. Measurements were taken at dawn at 12 locations within the site but outside the rain exclusion structure and averaged to plot LAI (1.5 ± 0.3 m2 m−2). Air temperature and humidity inside the chambers were used to simulate REW in the heat and heat-drought treatments, and 45% of incoming precipitation was withheld for simulations of REW in the drought and heat-drought treatments. Maximum extractable water in this soil type was assumed to be 120 mm (Soil Survey Staff, Natural Resources Conservation Service, United States Department of Agriculture, http://websoilsurvey.nrcs.usda.gov). Bulk density was measured at the site in July 2015 and was equal to 1.4 g cm−3. Simulations of REW with the BILJOU model were performed online (https://appgeodb.nancy.inra.fr/biljou/). REW was also simulated at a nearby site (∼10 km distant) that experienced significant tree mortality in 2002 (Breshears et al 2005, 2009) (figure 1) to determine the long-term soil moisture conditions and drought intensity experienced by trees in the study region. Simulations were conducted using yearly on-site measurements of LAI, 120 mm maximum extractable water in the soil and soil bulk density of 1.4 g cm−3.
Figure 1. Experimental drought and heat conditions surpass the 2002 drought that caused regional-scale mortality. (A) Relative extractable water (unitless) and (B) mean annual air temperature for the experimental treatments (2012–2016) relative to 1993–2006 observations at a nearby (∼10 km distant) site that experienced >90% pine and >25% juniper mortality in 2002 (Breshears et al 2005, 2009). The x-axes for the long-term plot (1993–2006) and for the experiment (2012–2016) are aligned so the most severe year of the regional drought in 2002 is aligned with the second year of experimental treatments (2013). In (B), the drought plot data are plotted but are hidden by the ambient plot data.
Download figure:
Standard image High-resolution imagePhysiological measurements and biotic assessments
In this paper we primarily report average values of all parameters; detailed temporal examinations of the data are available in (Garcia‐Forner et al 2016, Grossiord et al 2017a, 2017b, 2017c). Averages were calculated from campaign measurements conducted four to ten times per year. Brownness was surveyed using two people per date on every tree, and calculated after Gaylord et al (2013). NDVI was measured using FieldScout CM 1000 (Spectrum Technologies, Aurora, IL), held only in directly illuminated locations to minimize shading impacts, and was measured on three to five locations per tree and averaged. Leaf level stomatal conductance and photosynthesis was measured using the LI-6400 (Li-Cor, Lincoln NE;17) in the morning hours (typically 7:00–10:00 am) and was measured randomly across treatments and species to obtain comparable values (Garcia‐Forner et al 2016). Pre-dawn and mid-day leaf water potentials were averaged for two samples per tree per date, and measured using a Scholander-type pressure chamber (PMS, Corvallis, OR; Grossiord et al 2017a, 2017b). The iso/anisohydry slopes were then calculated from these measurements using annual datasets for each tree (Martínez‐Vilalta et al 2014). Twig level percentage loss of conductance (PLC) was calculated using the water potential measurements along with vulnerability curves developed on-site (Garcia‐Forner et al 2016). Sap flux density was measured using the thermal-dissipation method (Granier 1987; see Grossiord et al 2017c for detailed description of the sap flux method and calculations), and leaf-specific and canopy conductance were measured using a simplified inversion of the Penman-Monteith model (Monteith and Unsworth 1990). Whole-tree leaf area:sapwood area ratio (Al:As) was measured using allometrically scaled, destructive samples at the branch level for the target trees within each treatment (McBranch et al in press). Non-structural carbohydrates (NSCs) were sampled and processed per Dickman et al (2015), including annual measurements of foliage, twig, stem, coarse and fine roots. These NSC concentrations were then scaled for pine using the pine equations from Bond-Lamberty et al (2002) and the USFS Forest Inventory and Analysis generalized equations for juniper (Jenkins et al 2003). The depths of root water uptake for each species and treatment combination were determined using measurements of the water isotope ratios for the different depths of water sources as well as xylem water, and using standard mixing models to constrain the depths of water uptake (Grossiord et al 2017a).
Lindgren funnel traps were placed throughout each treatment and collected approximately monthly throughout the life of the project. Insects were sorted by family and grouped by trophic groups. All bark beetles were identified to genus using the insect collection at the Smithsonian Natural History Museum. All bark beetles collected were compared against the Ips confusus from the insect collection, confirming absence of Ips confusus at the site during the experimental period. Insect abundance is presented as the mean number of individuals trapped per number of days between samples and per number of traps on site, as is consistent with the insect trapping literature (e.g. Gaylord et al 2013). Tree attack rates were assessed via the methods of Gaylord et al (2013).
Modeling
We conducted simulations with the Terrestrial Regional Ecosystem Exchange Simulator (TREES; Mackay et al 2015). TREES is a hydraulically sophisticated ecosystem model that has been successfully used to assess drought responses in trees. TREES was run at half-hourly time steps using site-specific micrometeorological forcing (i.e. air temperature, VPD, photosynthetically active radiation, windspeed, precipitation, and soil temperature) spanning years 2012 through 2016. Input forcing air temperature, soil temperature, and precipitation for each treatment plot were modified to match timing and magnitude of the experimental treatments. Four simulations were run for each species using ambient, drought, heat, and heat + drought treatments. The TREES model was tuned for each species x treatment by matching simulated pre-dawn and mid-day water potentials to observations over the full five years. Parameters for photosynthesis were set using gas exchange data, and those for the hydraulics were set using observed sap flux, pre-dawn water potential, and mid-day water potential for one well-watered day. Although the mechanism and extent of xylem refilling is currently a matter of debate, we set the simulations to allow refilling of xylem during the monsoon in each simulated year. We used this approach because we have consistently observed a rapid recovery of plant water potential in pine after precipitation interrupts a prolonged drought period, during which leaf water potential remained higher than the soil, suggesting hydraulic isolation of the trees.
Initial rooting depths were established within the model based on site-specific information for the soil thickness, with shallow soil root depths (0–5 cm, 5–15 cm), deep soil depth (15–65 cm), and a bedrock root depth with an underlying permanent water source. Roots in the bedrock terminated at the top of the permanent water source, but within the capillary fringe, assuming a porous medium within bedrock fractures. This ensured that bedrock roots were exposed to steady-state equivalent soil water content of about 60% of porosity. The proportion of absorbing root area in the bedrock was calibrated in the ambient plots so that simulated water potentials matched observations. Juniper was satisfied with 15% of its root area in the bedrock, while pinon required only 10% of its root area in bedrock. The same root configurations by species allowed simulated water potentials to match observations in each of the treatment plots without further calibration (see results).
Statistical analyses
All analyses were performed using the software R (3.2.1, R Development Core Team 2015) with α = 0.05 to determine statistical significance. We analyzed responses of mean yearly pre-dawn leaf water potential (ΨPD, April–August, 2012–2016), mean yearly stomatal conductance and photosynthesis (April–August 2012–2016), whole-tree NSC (i.e. June sampling 2012–2016), leaf-specific hydraulic conductivity (g m−1 s−1 MPa−1, sampled once in September 2016), mean daily canopy-level stomatal conductance (mmol m−2 s−1, March–September 2016), whole-tree Al:As (i.e. sampled once in August 2016), mean yearly NDVI and brownness (2012–2016) of each species to precipitation reduction, atmospheric warming and the combination of the treatments using mixed linear, random intercepts models where warming (yes or no), drought (yes or no) and their interaction were used as fixed effects. For all tests, the individual trees nested in the chambers were input as random effects. R-square (r2) was obtained for linear mixed effects models following Nakagawa and Schielzeth (2013) and adapted by Jon Lefcheck (http://jonlefcheck.net/2013/03/13/r2-for-linear-mixed-effects-models/). Statistical analyses were performed using the package nlme for Linear mixed effects models.
Results
The experimental manipulations successfully reduced precipitation and REW, and elevated temperature, over the five years of treatment. We present these results in figure 1 in contrast to the historic drought that killed trees in this region; the manipulations along with regional climate caused conditions between 2012–2016 that were more severe than those of the 2002–2003 drought that killed up to 90% of pines and 25% of junipers locally (figure 1; Breshears et al 2005, 2009, Garrity et al 2013).
Despite the severe experimental treatments, no trees of either species died. The percentage brown foliage never exceeded 12% for either species (p > 0.05 for all treatments compared to ambient; figure 2(A); see SI2 for full statistical results); far below the threshold of >50%–90% for mortality observed for these species (Breshears et al 2009, Gaylord et al 2013). NDVI, another metric of canopy health, likewise showed no treatment impacts (p > 0.18; figure 2(B)). Stomatal conductance and photosynthesis (p < 0.05 for drought in juniper and all treatments in pine), leaf-specific hydraulic conductivity (p < 0.05 for heated juniper and droughted pine), and canopy conductance (p < 0.05 for drought for both species; figures 2(C)–(E), (G)) all showed treatment impacts but none were as strongly impacted as has been observed prior to mortality of these species (Pangle et al 2012, Plaut et al 2013). The mid-day to pre-dawn water potential slope, a metric of iso/anisohydry, showed no change in response to the treatments (p > 0.85; figure 2(F)), nor did whole-tree leaf area:sapwood area ratios (p > 0.20; figure 2(H), consistent with twig-measurements Grossiord et al 2017b). Based on these results (figure 2), it appears that the treatment impacts on leaf and whole-tree level physiology were relatively minor despite the severe experimental treatments (figure 1).
Figure 2. Minor physiological and structural responses to drought and heat. Average (2012–2016) (A) crown percent brown foliage, (B) NDVI, (C) stomatal conductance, (D) photosynthesis, (E) leaf-specific hydraulic conductivity, (F) the slope of mid-day to pre-dawn water potential (a metric of iso/anisohydry), (G) canopy conductance, and (H) whole-tree leaf area:sapwood area ratio for pine and juniper. (H) was measured in 2016 only. Statistical tests are provided in the main text and statistical details are provided in the SI.
Download figure:
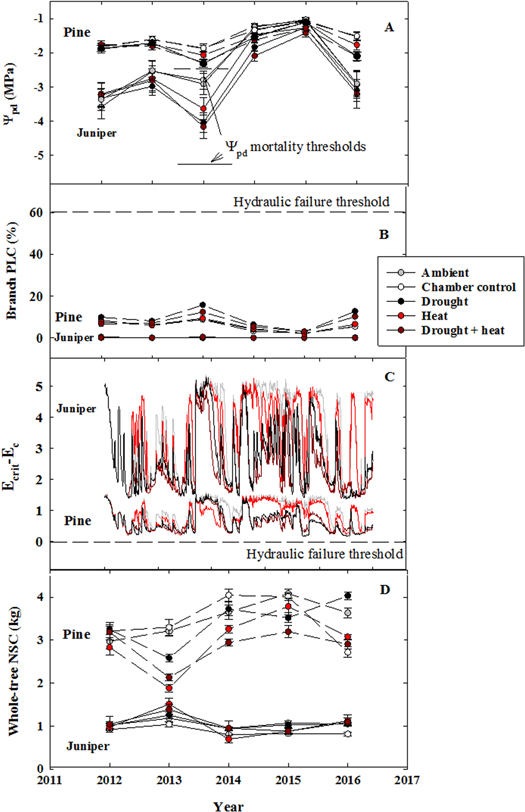
Standard image High-resolution imageConsistent with the small experimentally-induced shifts in physiology (figure 2), there was little evidence that the droughted and heated trees approached thresholds for carbon starvation or for hydraulic failure (figure 3). The April–August average Ψpd did not exceed the previously determined mortality thresholds for these species (figure 3(A); McDowell et al 2016). There were significant treatment effects for heated juniper and heated and droughted pine (p < 0.05) but these trees showed little apparent damage to their crowns (figures 2(A) and (B)). Branch level PLC, calculated using Ψmd and on-site vulnerability curves showed that neither species exceeded 20% PLC, far below the posited mortality threshold of ≥60% (McDowell et al 2013, Anderegg et al 2015, Sperry and Love 2015, Adams et al 2017b; figure 3(B)). Modeled transpiration rates relative to the species-specific critical rates to induce embolism (Ecrit–Ec) never fell below zero, again suggesting that significant hydraulic failure did not occur in these trees (figure 3(C); Sperry and Love 2015). Finally, whole-tree non-structural carbohydrate mass (NSC; concentration values scaled with allometric equations) showed a significant treatment impact for heated and heat plus drought pine trees (p = 0.01) but never fell dramatically below ambient levels (figure 3(D)).
Figure 3. Minor evidence of mortality risk, hydraulic failure, and carbon starvation. Shown are 2012–2016 (A) April–August mean pre-dawn water potential, which never exceeded the mortality threshold values of −2.4 MPa and −5.3 MPa for pine and juniper, respectively, (B) branch level percentage loss of conductivity calculated using the pre-dawn water potentials and on-site vulnerability curves, (C) TREES modeled critical transpiration rate minus actual transpiration rates, and (D) measured whole-tree non-structural carbohydrate mass. Each panel highlights that neither species approached hydraulic or carbohydrate thresholds for mortality over 2012–2016. (A) Trees approached (in pine) but did not exceed previously identified thresholds for mortality (McDowell et al 2016). (B) Trees did not approach the previously identified PLC threshold for mortality of >60%. (C) Trees did not approach the critical transpiration thresholds (e.g. negative values in Panel C) associated with hydraulic failure. (D) Few treatment impacts, or declines, in whole-tree non-structural carbohydrates were observed for either species (exception heated and droughted and heated pine).
Download figure:
Standard image High-resolution imageTwo landscape-scale mechanisms of tree persistence under drought and heat were supported. First, water isotope measurements and model simulations both showed that juniper and pine acquired water from below the bedrock surface throughout the experimental period (figures 4(A) and (B)). Specifically, comparison of tree xylem water isotope ratios with soil water isotope ratios revealed consistent tree use of bedrock water (figure 4(A)) despite variation across seasons and treatments (Grossiord et al 2017a). Consistent with the isotope results, TREES model simulations were unable to reproduce observed water potentials without inclusion of rooting access to a bedrock water source (figures 4(B), 5 and 6). Bedrock access had a large impact on mortality likelihood, as simulations with no bedrock access reached lower whole-tree hydraulic safety margins and had higher PLC more frequently than simulations with bedrock access (figures 5 and 6). Thus, two independent lines of evidence suggest that access to bedrock water appears to buffer the trees from severe drought and heat impacts and may be responsible for the trees not exceeding the thresholds associated with mortality (figure 3).
Figure 4. Deep water is a mechanism of persistence under drought and heat. (A) Deep water contribution to xylem water using natural abundance water isotope sampling for 2013–2015, and (B) TREES simulations of deep water contribution to transpiration. Both (A) and (B) suggest both species had persistent access to deep water. The depth to bedrock in this ecosystem is ∼40–80 cm. (A) Re-calculated from Grossiord et al (2017a). See Grossiord et al (2017a) for detailed analyses of treatment impacts on bedrock water use.
Download figure:
Standard image High-resolution imageFigure 5. Bedrock water access improves piñon model predictions. Comparison of pine trees observed versus modeled pre-dawn (upper panel) and mid-day water potentials (lower panel) when TREES incorporates a sub-bedrock water sources (black symbols) versus when TREES assumes all water uptake is above the bedrock (gray symbols). The improved model predictions when including bedrock water lends support to the conclusion that these trees had access to bedrock water.
Download figure:
Standard image High-resolution imageFigure 6. Bedrock water access improves juniper model predictions. Comparison of juniper trees observed versus modeled pre-dawn (upper panel) and mid-day water potentials (lower panel) when TREES incorporates a sub-bedrock water sources (black symbols) versus when TREES assumes all water uptake is above the bedrock (gray symbols). The improved model predictions when including bedrock water lends support to the conclusion that these trees had access to bedrock water.
Download figure:
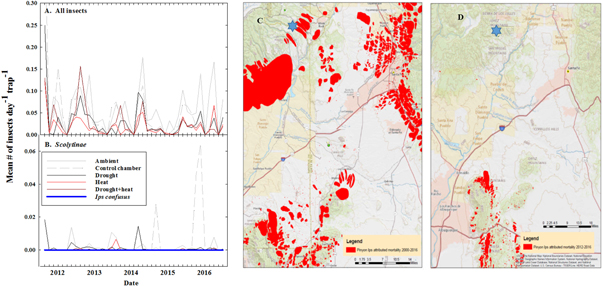
Standard image High-resolution imageA second potential mechanism of piñon persistence may have occurred at this site. Insect trapping showed abundant insect presence at the site (figure 7(A)) but few Scolytinae (bark beetles) were trapped (figures 7(C) and (D)). This indicates that while insects were generally abundant, those that attack these tree species (Scolytinae) were notably absent. Most noteworthy, the bark beetle species that kills piñon pine, Ips confusus (LeConte), was never trapped on site (figure 7(B)). Likewise, no evidence of bark beetle attack on the tree bark was observed (Online table 1). These observations are consistent with the lack of Ips confusus hosts (live piñon pine) in the surrounding region (figures 7(C) and (D)). The 2002 drought removed the vast majority of mature piñon pine from the local landscape (figure 7(C)) and subsequently there was virtually no detected presence of insect attacks in the region of the field site (via aerial detection; USDA Forest Service, 2018), even during the particularly severe regional drought of 2013 (figure 7(D)). This explanation applies only to piñon, not juniper, at this site.
Figure 7. Refugia from insect attack promotes resiliency to drought and heat. Left-hand column shows observations of trapped insects from the experimental site. (A) All insects show that a large number of insects were trapped at this site. In contrast, (B) Scolytinae were rarely observed in any treatments. The insects that kill piñon pine, namely Ips confusus, were never observed (blue line in panel B). (C), (D) aerially-detected piñon pine mortality attributed to Ips confusus from 2000–2016 (panel C) from 2012–2016 (panel D). The field site is shown in with a blue star near the top of the map. From (C), (D) it is apparent that the mortality event of 2000–2003 removed a large portion of piñon from the landscape surrounding the field site, creating an island of refugium from Ips confusus host trees. (C), (D) is consistent with local mortality observations (Breshears et al 2005, 2009, Garrity et al 2013).
Download figure:
Standard image High-resolution imageDiscussion
Our test of tree persistence under experimental hotter-drought demonstrates that these species can withstand particularly severe conditions (figure 1) when they have access to a quasi-permanent source of water (figure 4), relative to that expected based on prior observations and experiments on these species (e.g. Breshears et al 2005, Adams et al 2009, Plaut et al 2012). These results are consistent with our hypothesis that resistance to drought and heat would be associated with minimal carbon starvation or hydraulic failure (figure 3) and greater belowground water uptake (figures 4–6). We also observed support for the additional mechanism of isolation of trees from attacking insects (figure 7).
Initially, the lack of strong physiological impacts or mortality (figures 2 and 3) was surprising given that the treatment effects on soil moisture and temperature (figure 1) were more severe than those that resulted in widespread mortality of these species previously in this same region, both during regional drought (Breshears et al 2005, 2009, Garrity et al 2013) and experimental drought (Pangle et al 2012, Plaut et al 2013, Gaylord et al 2013). The resistance of these trees to severe drought and heat treatments appears to originate with their access to water in the bedrock fractures in this area (Newman et al 1997), which provides a water source sufficient to maintain gas exchange even under severe conditions (figures 4–6; SI figures 2, 3, consistent with Schwinning 2010, Klos et al 2018, and Rempe and Dietrich 2018). This presence of quasi-permanent water may have promoted survival of this stand of trees during the 2002 drought and associated regional die-off (figure 7), and appears to have further promoted survival of the extreme drought and heat treatments we imposed in 2012–2016 (figure 1). Whole-tree PLC was significantly higher than twig level PLC (as has been observed before in the southwestern USA e.g. Johnson et al 2018), yet the presence of bedrock water significantly increased transpiration such that plants maintained functional gas exchange even under severe drought and heat. Both pine and juniper increased water uptake from the bedrock source under drought treatment (Grossiord et al 2017a).
The availability of bedrock water may have promoted this refugiums isolation from insects. This stand of trees is isolated from other piñon pine trees in particular (Breshears et al 2005, 2009, Garrity et al 2013; and figures 7(C), (D)), thus there are few piñon trees to host Ips confusus. Beyond our observation of zero pinon ips trapped at the site (using the standard technique; Lindgren 1983, Gaylord et al 2013), this isolation argument is supported by the known, and very short, active flight distances of bark beetles (Evenden et al 2014, Kees et al 2017), coupled with the extraordinary rarity of long flight distances when insects are entrained in aeolian dispersal (Safranyik et al 1992, Jackson et al 2008, de la Giroday et al 2012). Even if surviving (undetected) insects were in the region, there was likely to be high resistance to colonization of these trees by I. confusus given the relative minor stress our experimental trees endured (figure 2). Thus, bedrock water sources may ultimately have promoted this stand's survival from biotic insect attack, thus providing evidence of hydraulic refugia (McLaughlin et al 2017), with a subsequent feedback through isolation of the refugium from attacking insects.
It is possible that the trees would have survived insect attack if it had occurred, as they had no evidence of significant carbon starvation or hydraulic failure (figure 3), which would suggest they may have had sufficient defensive capacity had Ips confusus insects attacked. Thus we cannot conclude that the absence of insects allowed survival, but only that the absence of insects is an additional potential mechanism of persistence under drought and heat (consistent with García de la Serrana et al 2015). While thus speculative, the isolation of live trees from nearby conspecific hosts that carry attacking insects constitutes a potentially strong negative feedback on insect outbreak likelihood, by which outbreak likelihood is reduced via the decimation of host trees during a prior outbreak. This is a logical outcome of the spatial patterns of insect outbreaks, but not one that had previously been quantified empirically, nor under conditions of experimental warming (Logan et al 1998, Hart et al 2015). This negative feedback mechanism of forest resistance to climate-change-type-drought has similarities to fire-refugia, in which fires are less likely to reach remnant stands of trees due to the lack of fuel (Schoennagel et al 2009). Thus a prior beetle outbreak has reduced the abundance of live insect hosts in the vicinity, reducing exposure to bark beetle attack within the remnant stand during subsequent droughts.
Determining the regional frequency of forests that have access to quasi-permanent belowground water sources, and that are isolated from attacking insects, are large observational challenges we must consider before Earth system models can integrate these mechanisms into forecasts. For belowground water sources, we must know the overlap between vegetation rooting depths and quasi-permanent water store depths across regions. The creation of isolated stands of ponderosa pine (Pinus ponderosa) throughout the same landscape as our study site, during a severe regional mortality event in the 1950s, was associated with co-location of stands on deep soils (Allen and Breshears 1998), providing further support for the role of rooting depth or access to deeper soil water as a mechanism of persistence in this region. Promising datasets on water table depths are emerging (e.g. Pelletier et al 2016) but they are not yet available at sufficiently fine-scale to match the existing distribution of topography and vegetation in many landscapes. Likewise, in the absence of a local endemic population of tree-killing insects in an isolated forest stand, its likelihood of experiencing mortality due to insect attack is a function of the severity and proximity of the nearest ongoing outbreak of forest insects (Aukema et al 2008, de la Giroday et al 2012). Thus, while the mechanisms of persistence that we identified are clearly important at our site, it will be a larger challenge to ascertain how frequent these mechanisms manifest at landscape to regional scales.
We provide evidence of two-interdependent mechanisms of resistance of trees under futuristic conditions (Keppel et al 2012), in this case, the coupled hydraulic refugia and biotic isolation mechanisms. However, multiple questions remain. Our site is located at the upper elevation (wetter) ecotone for piñon pine, and experienced a large rain event in September 2013, both of which may be reasons why the bedrock had a significant amount of water to promote survival in subsequent years. Understanding interactions of mortality with landscape position and climate variability remains a challenge for this species of pine (Meddens et al 2015), despite the fact that this is among the best-studied plant species globally in regard to drought-associated mortality (Breshears et al 2018). Furthermore, some belowground water sources are ephemeral and thus will not necessarily sustain refugium into the future. Elevated CO2 was not mimicked in our experiment, but this appears to have no impact on mortality likelihood under heat and drought (Duan et al 2014, Allen et al 2015). Lastly, this could be a selection event in which the remaining stand is adaptively predisposed to survive (Gutschick and BassiriRad 2003); testing this idea will require following forest refugia through multiple drought events.
Conclusions
Our results suggest trees may be particularly resistant to severe drought and heat if they have access to a quasi-permanent source of soil water, providing some optimism regarding the potential survival of trees under a changing climate relative to predictions that do not incorporate these mechanisms of persistence (McDowell et al 2016). The dual mechanisms of access to bedrock water and avoidance of subsequent insect outbreaks may mitigate the impacts of hotter droughts, promoting the perpetuation of forest patches in landscapes that have experienced widespread tree die-off. Incorporating the presence and mechanisms of forest resistance to climate-change-type-drought into earth system models may prove valuable to improve the predictive accuracy of future forest loss (Bonan and Doney 2018) and should be considered as we test the mechanisms of future survival (Lloret et al 2012). However to accomplish this we must understand the distribution of vegetation rooting depths relative to quasi-permanent sources of soil water, which is a large challenge. Because these hydraulic (Mclaughlin et al 2017) and insect refugia may be critical for the persistence of trees under a warming climate (Keppel et al 2012, Sanchez-Salguero et al 2017), preservation of refugium may be a strategic management choice to maximize species conservation into the future.
Acknowledgments
This project was supported by the Department of Energy, Office of Science, and Pacific Northwest National Lab's LDRD program. DDB participation was supported via NSF EF-1340624; EF-1550756, and EAR-1331408, DEB-1824796 and DEB-1833502. CG was supported by a Director's Fellowship from the Los Alamos National Laboratory and by the Swiss National Science Foundation SNF (PZ00P3_174068). AV was supported by a fellowship from Generalitat Valenciana (BEST/2016/289) and the project Survive-2 (CGL2015-69773-C2-2-P MINECO/FEDER) from the Spanish Government. DSM was supported via NSF IOS-1450679, IOS-1444571, and IOS-1547796. We appreciate the field assistance of Heath Powers in construction of the experiment and the guidance of Paul Hanson, Stan Wullschleger, and Rich Norby in the design of the experiment. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the US Government.
Authorship:
All authors contributed to the writing. In addition, NGM led the project, conducted field work, and drafted the manuscript, CG conducted field and lab work and statistical analyses, HDA conducted field and lab work, SP contributed to field and lab work and advised on insect ecology, DSM led the modeling, DDB assisted with the manuscript, CDA assisted with the manuscript, IB conducted field and lab work, LTD conducted field and lab work, MG assisted with the remotely sensed imagery of insect outbreaks, NM conducted field and lab work, WTP assisted with the manuscript, AV conducted field work, BA advised on insect ecology, DG advised on insect ecology, CX assisted with the manuscript.
Data accessibility:
Most of the data associated with this manuscript is housed on the US Department of Energy's Office of Science and Technical Information website, e.g. https://osti.gov/biblio/1454272. Data in the format of that presented in this paper will be housed there upon acceptance of the manuscript.
This draft manuscript is distributed solely for purposes of scientific peer review. Its content is deliberative and predecisional, so it must not be disclosed or released by reviewers. Because the manuscript has not yet been approved for publication by the US Geological Survey (USGS), it does not represent any official USGS finding or policy.