Abstract
The reconstruction of segmental bone defects remains an urgent problem in the orthopaedic field, and bone morphogenetic protein-2 (BMP-2) is known for its potent osteoinductive properties in bone regeneration. In this study, chitosan microspheres (CMs) were prepared and combined with absorbable collagen sponge to maintain controlled-release recombinant human bone morphogenetic protein-2 (rhBMP-2). The rhBMP-2-loaded composite scaffolds were implanted into 15 mm radius defects of rabbits and the bone-repair ability was evaluated systematically. CMs were spherical in shape and had a polyporous surface, according to SEM images. The complex scaffold exhibited an ideal releasing profile in vitro. The micro-computed tomographic analysis revealed that the rhBMP-2-loaded composite scaffold not only bridged the defects as early as 4 weeks, but also healed the defects and presented recanalization of the bone-marrow cavity at 12 weeks. These results were confirmed by x-ray. When compared with other control groups, the composite scaffold group remarkably enhanced new bone formation and mechanical properties, as evidenced by bone mineral content evaluation, histological observations and biomechanical testing. Moreover, the biocompatibility and appropriate degradation of the composite scaffold could be obtained. All of these results clearly demonstrated that the composite scaffold is a promising carrier of BMP-2 for the treatment of segmental bone defects.
Export citation and abstract BibTeX RIS
1. Introduction
Because bone tissue generally possesses an excellent ability to regenerate spontaneously, small defects can be healed basically by self-repair. Unfortunately, large bone defects caused by trauma, tumors, congenital malformation or corrective osteotomies always result in delayed union or non-union of bone. This regeneration remains a challenging clinical problem in orthopaedic surgery. Currently, bone graft substitutes including autologous, allogeneic bone and prosthetic implants are chosen to help healing in clinical therapy [1]. In addition, autogenous bone grafts have long been considered the gold standard for bone grafts due to their excellent osteoconductivity, osteoinductivity and osteogenicity [2]. Limitations and disadvantages to autografting exist, however, including insufficient autogenous bone, no adequate donor site and donor site morbidity [3, 4]. In view of the above grafts being unsatisfactory, it is imperative to explore much more convenient and effective treatment methods for bone defects.
Bone morphogenetic proteins (BMPs) form a subgroup of the transforming growth factor-β superfamily, which is a large group of proteins that affect cell growth, migration and differentiation, and play a regulatory role in tissue homeostasis and repair in adult and organisms [5]. Among the BMPs, BMP-2 is most extensively researched [6, 7] and has very strong osteoinductive activity [8]. Since 2002, the US Food and Drug Administration (FDA) and the European Medicines Agency have approved the clinical use of BMP-2 in certain spinal fusions, tibial fractures and dental grafts [9].
Despite its promising therapy, the clinical use of BMP-2 still requires great improvement, especially with respect to its delivering system. It is not currently feasible to apply recombinant human BMP-2 (rhBMP-2) alone into bone defects due to its rapid degradation and short half-life in vivo [7]. To overcome these problems, a number of carriers of rhBMP-2, including natural and synthetic biomaterials, have been explored in the past decade. Biomaterials are needed as carriers to maintain BMP retention at the defect site and provide a structural matrix for bone growth. Additionally, they may be used to provide controled and sustained delivery of BMP to mimic its temporal profile during natural bone healing in vivo [10]. Although progress has been made, an effective carrier of rhBMP-2 has not yet been found [7].
The formulation of BMP-2 that is currently approved uses an absorbable collagen sponge (ACS) as a carrier agent [9, 11]. In general, ACS was used to soak rhBMP-2, and the study indicated that rhBMP-2 did bind to the collagen sponge [12]. This combination of ACS and rhBMP-2 facilitates surgical implantation and rhBMP-2 retention at the treatment site [13]. However, it has the disadvantage of a fast degradation rate and low mechanical stability, resulting in a bolus release in the first several hours and an inability to maintain sustained and controlled release in vivo. Beyond influencing the regeneration effect, this formulation is only effective if BMP-2 is administered at much higher concentration than physiological needs, thus, raising concerns regarding safety.
Sustained delivery can be established through different retention mechanisms including encapsulation, absorption and complexation. Microspheres fabricated by either synthetic or natural materials have been commonly studied for controled delivery of BMP-2. However, proteins might be exposed to various organic solvents, nonphysiologic temperature variations and undesirable pH value during the procedure [14], which may denature the growth factor completely or adversely reduce its bioactivity.
In this study, a composite rhBMP-2 carrier was developed for segmental bone repairs. In our delivery system, the composite scaffold, which was generated, consists of ACS and chitosan microspheres (CMs). We chose ACS as the matrix because it is close to clinical application. In order to improve the initial burst of rhBMP-2 from ACS, CMs with porous structure were prepared to entrap rhBMP-2 using gentle, safe and practical tactics, avoiding the loss of bioactivity. Being a kind of hydrophilic cationic polysaccharide in nature, chitosan (CS) has been developed for a variety of biomedical applications, such as wound healing, drug delivery and orthopedics [15–18]. It possesses superior biocompatibility, biodegradability, non-toxicity and adsorption properties.
Additionally, CMs have been embedded within ACS scaffolds for the purposes of bone repair in segmental defects. The releasing behavior of the composite formulation was investigated. Then, a critical-sized defect model was created in a rabbit to evaluate the healing capacity of long bone defects. We hypothesized that the ACS combined with CMs was a superior delivery system of rhBMP-2 for enhancing in vivo osteogenic efficacy as compared to ACS alone.
2. Materials and methods
2.1. Materials
CS (Mw = 9.0 × 105, degree of deacetylation = 95%) was obtained from Beijing Chemical Reagents Co. (China). Bovine serum albumin (BSA) was purchased from Yuanju Bio-tech Co., Ltd (China). ACP was obtained from Jinling Medicine Co., Ltd (China). rhBMP-2 was kindly provided by Rebone Co. (China). All other reagents and solvents used were reagent grade.
2.2. Chitosan microspheres preparation and characterization
CMs were prepared using an emulsion-chemical cross-linking method [19]. Briefly, 0.6 g of CS was dissolved in 20 mL of 2% (v/v) aqueous acetic acid solution under magnetic stirring at room temperature until the solution was transparent. 3% of the CS solution was then filtered to remove sundries, and it was allowed to stand. 20 mL of the CS solution was uninterruptedly added into 100 mL of liquid paraffin containing surfactant 3% (v/v) Span 80 and Tween 80 (2:1), and agitated mechanically for 2 h at room temperature. 20 mL of 20% (v/v) vitriol solution was further added dropwise to the emulsion and stirred for another 4 h to stabilize the microspheres as a cross-linking agent. The microspheres were precipitated in the mixture solvent after repeated washing with excess amount of acetone. Then the resulting precipitations were repeatedly washed with distilled deionized water and treated with 4% (w/v) NaOH solution for 2 h. The microspheres were dialyzed for 3 days, prior to lyophilization. For comparison, CMs without polyporous structure were prepared according to the patent (CN 1203119C) [20].
The microstructure of the CMs was observed through a scanning electron microscope (SEM, QUANTA 250, FEI). Prior to SEM analysis, dried microspheres were uniformly sputter coated with gold under vacuum.
In the adsorption study, BSA was selected as the model protein and CMs without polyporous structure were used as the control group. 50 mg of CMs with or without polyporous structure were added into 50 mL of 1 mg mL−1 BSA solution. Then the adsorption experiments were conducted on a shaking incubator (80 rpm) at 37 °C. After sufficient adsorption, the residual BSA concentration in the supernatant was determined with a UV-visible spectrophotometer at 595 nm using a Bradford Protein Assay Kit (Beyotime, China). All experiments were performed in triplicate for each sample.
2.3. Scaffolds fabrication
The fabrication of scaffolds was carried out under sterile conditions. In this study, ACS was used and its size was 3 cm × 1.5 cm × 0.5 cm. Three different scaffolds were prepared as follows: (1) ACS alone, (2) 100 µL rhBMP-2 (0.5 µg µL−1) was added onto the ACS, (3) 1 mg of CMs was added into 100 µL of rhBMP-2 solution (0.5 µg µL−1) to absorb protein and then transferred to the ACS; the beads were then spread on ACS. All scaffolds were rolled into column shapes after they were freeze-dried, and stored at 4 °C for future use under sterile conditions.
2.4. In vitro release study
The CMs were added into 0.5 mg mL−1 rhBMP-2 solution to adsorb rhBMP-2 for 3 h, and then the rhBMP-2-loaded CMs were obtained after lyophilization. The rhBMP-2-loaded ACS was prepared using the method described previously.
The rhBMP-2 released from three different samples is as follows: (1) 2.5 mg rhBMP-2 was loaded onto ACS, (2) 50 mg of the rhBMP-2-loaded CMs, which contain about 2.5 mg rhBMP-2, (3) 50 mg of the rhBMP-2-loaded CMs containing 2.5 mg rhBMP-2 were transferred to ACS and tied up, avoiding scatter of CMs. Three different samples were dispersed into 5 mL of phosphate buffered saline (PBS, pH 7.4) and kept in a shaking incubator (80 rpm) at 37 °C. At preset time intervals, the samples were centrifuged, and the 2 mL of the supernatant was withdrawn and replaced with the same amount of fresh PBS buffer. The amount of BMP released from the scaffolds was evaluated by a Bradford Protein Assay Kit (Beyotime, China). All experiments were performed in triplicate for each sample.
2.5. Segmental defect induction and surgical implantation of the scaffolds
All surgical procedures were reviewed and approved by the Institutional Animal Care and Use Committee. Seventy-two male New Zealand white rabbits weighing about 3 kg were used for the study. Sixty-nine rabbits were randomly divided into three groups: (A) ACS group, (B) rhBMP-2/ACS group, (C) rhBMP-2/ACS+CMs group; the other three rabbits were used as the normal control group. Animal surgery was carried out under sterile conditions. Before operation, the rabbits were weighed and anesthetized by 3% pentobarbital sodium (30 mg kg−1) intramuscularly. The right forelimb of the rabbit was shaved before operation. A longitudinal skin and musculature incision measuring approximately 30 mm was made at the anteromedial aspect. A unilateral segment of the periosteum with a radius of 15 mm in length was removed using a hand drill in the middle of the radius. The defect site was subsequently irrigated with normal saline, and filled with the scaffolds described above. The underlying musculature and the skin were closed, and then the wound was covered with sterile gauze. After the surgery, the rabbits were individually caged and normally fed. The rabbits were sacrificed successively at time intervals of 2, 4, 8 and 12 weeks. Each respective series consisted of five animals, with the exception of three animals at 12 weeks for the mechanical test.
2.6. Radiographic examination
The harvested bone specimens at 4, 8 and 12 weeks were examined by x-ray machine (WDM, China) at 46 KV and 100 mA with an integration time of 40 ms, to evaluate new bone formation in the bone defects.
2.7. Micro-computed tomographic evaluation
The defective radius and adjoining ulna were harvested and scanned with a micro-computed tomographic (CT) imaging system (GE, explore Locus SP micro-CT). Three-dimensional CT images of the radius and ulna were reconstructed using the Microview software to evaluate the repairing process for the defect site and analyzed with ABA analysis software to calculate the bone mineral content (BMC) and bone mineral density (BMD) of the bone defects.
2.8. Histological assessment
All of the harvested bone specimens were fixed in 4% neutral formaldehyde and rinsed overnight with running water. The samples of bone tissue were then dehydrated through graded ethanol, cleared in xylene, and decalcified with 12.5% EDTA. After decalcification, the resultant samples were embedded in paraffin wax and tissues were sectioned at 5 µm.
The paraffin sections were stained with hematoxylin and eosin and Masson's trichrome staining for histological assessment. Specimens were observed with a light microscope (TE2000-U, Nikon) under 100× magnifications.
2.9. Biomechanical test
To evaluate the mechanical stability of the regenerated bones, the defective radius and adjoining ulna removed at week 12 were subjected to the three-point bending test. Before testing, the specimens were thawed at 4 °C for 24 h and then soft tissue was carefully dissected from the limbs. The intact radius in company with the contra-lateral ulna was tested at ambient temperature and humidity using a mechanical testing machine (HY-0230, Shanghai, China). Briefly, the specimens were positioned on two supports spaced 8 mm apart, and the bending load was applied at the midpoint of the defect at a constant displacement of 5 mm min−1 to break. During the bending measurement, the data was automatically recorded and stored using specialized software associated with the HE machine. At least three replicates were carried out for each group, and the results were expressed as means ± standard deviation (mean ± SD).
2.10. Statistical analysis
All data were expressed as the mean ± SD and statistical analyses were performed using one-way ANOVA. Comparison between the two groups was made using the Tukey post-hoc test with statistical significance evaluated at p < 0.05.
3. Results
3.1. SEM of chitosan microspheres
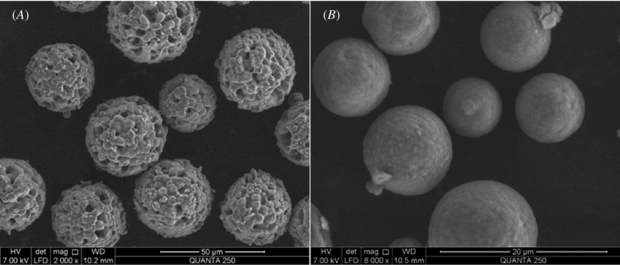
The shape and surface microstructures of the CMs are shown in figure 1. It was notable that the microspheres were spherical in shape. Compared to the ordinary compact microspheres (figure 1(B)), the CMs prepared in the study displayed a loose topography with the polyporous structure (figure 1(A)).
Figure 1. SEM images of CMs with polyporous structure (A) and compact CMs prepared via ordinary procedure (B).
Download figure:
Standard image3.2. Model protein adsorption on the chitosan microspheres
The BSA adsorption capacity of the CMs with the polyporous structure was 716.3 mg g−1, while the BSA adsorption capacity of CMs without the polyporous structure was only 543.9 mg g−1.
3.3. In vitro rhBMP-2 release
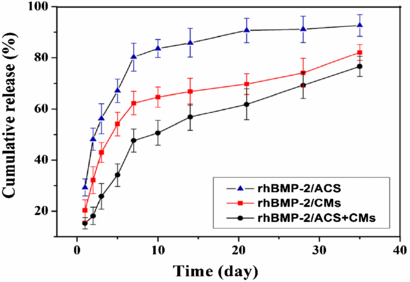
The rhBMP-2 release characteristics of the three different scaffolds are summarized in the percentage cumulative release profiles shown in figure 2.
Figure 2. Cumulative release profiles of rhBMP-2 from three different scaffolds in PBS for 35 days.
Download figure:
Standard imageThe rhBMP-2/ACS group showed a notable initial burst within the first 7 days (80.32%), and then was released slowly reaching 92.71% at 35 days. A burst release in the early stage was also observed in the rhBMP-2/CMs group, and was followed by continuous release. The amount of rhBMP-2 released after the initial 7 days was 62.27%, and 82.13% of rhBMP-2 released until the 35th day. In general, the release rate of rhBMP-2 from CMs was lower than that from ACS, indicating that CMs exhibited controlled release of rhBMP-2. The rhBMP-2/ACS+CMs group showed minimal burst release followed by moderate release over the subsequent phases. The lower level release was benefited by the rhBMP-2-loaded CMs wrapped in ACS materials. The amount of rhBMP-2 released from the composites in PBS was only 47.63% after 7 days and 76.67% after 35 days. The sudden release from 5 to 7 days may be explained by the swelling of ACS in PBS.
3.4. Radiographic analysis
Qualitative assessment of x-ray radiographs demonstrated new bone formations for the three groups with different implants (figure 3).
Figure 3. Representative serial radiological images of the segmental radius at 4, 8 and 12 weeks post-operation. Group A (ACS alone), group B (rhBMP-2/ACS implants) and group C (rhBMP-2/ACS+CMs implants).
Download figure:
Standard imageIn the control group (group A), little radiopacity appeared in the defects at 4 weeks after the operation, and observable callus formation was seen in the distal ends of the defects at 8 weeks. It seemed that both ends of the bone defects were partially bridged at 12 weeks, suggesting bad treatment efficacy of ACS.
Groups B and C had better performance over group A. At 8 weeks, bridging callus formations were observed and consolidated thereafter. Although full union of bone defects in groups B and C was seen at 12 weeks, the bone defects of group B seemed to remain unrepaired while the bone defects of group C appeared to be completely healed. Moreover, group C showed better regeneration efficacy visibly compared with group B both at 8 and 12 weeks post implantation.
3.5. Micro-CT analysis
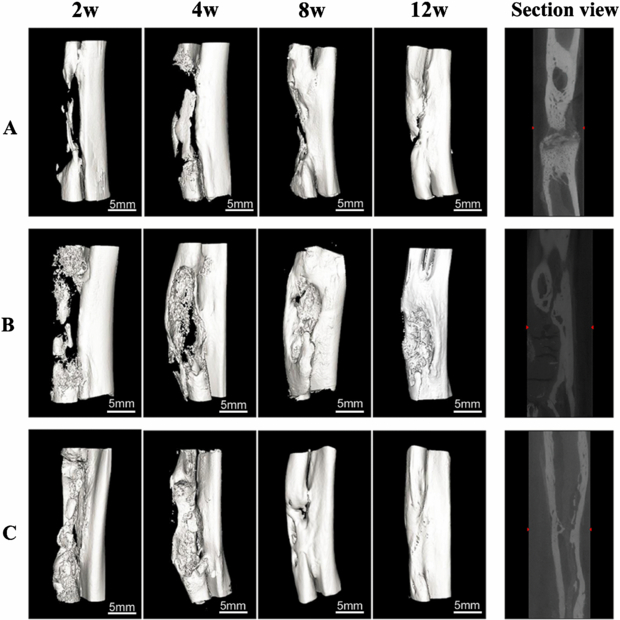
The healing process of the long segmental defected bone in each group was clearly seen from 3D reconstruction images (figure 4). Just like the results of x-ray evaluation, group A showed minimal bone formation, and massive callus was formed up to eight weeks. Both ends of the bone defects did not bridge completely, resulting in the nonunion of bone. In group B, more neo-bone formation was present in the bone defects as compared with group A throughout the process. Bridging callus formations were detected as early as 8 weeks; however, the defected bone seemed to be not healed completely at 12 weeks. Group C exhibited remarkable bone bridging at 4 weeks and excellent healing capacity of bone. In addition, the bone defects seemed to be almost fully repaired at 12 weeks. As it can be seen from the sectional view images, only group C achieved recanalization of the medullary cavity, suggesting the complete repair of bone defects.
Figure 4. Three-dimensional micro-CT reconstruction of the bone regeneration in the radius defect from the animals at the different stages of the bone reconstruction process; the right figure shows the section view of the defected radius site at 12 weeks. Group A (ACS alone), group B (rhBMP-2/ACS implants) and group C (rhBMP-2/ACS+CMs implants).
Download figure:
Standard image3.6. BMC and BMD
The BMC and BMD were measured by the bone analysis function of the ABA analysis software. The BMC indicates the total BMC in the selected region of interest (ROI) and BMD denotes the ratio of BMC to the volume of the selected ROI.
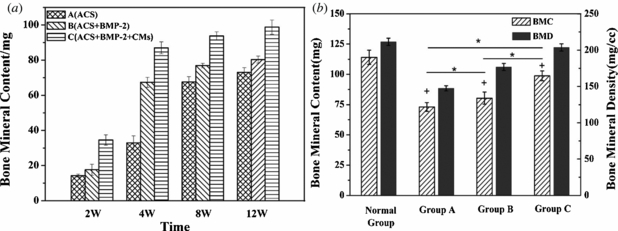
Figure 5(a) compares the BMC of three groups at 2, 4, 8 and 12 weeks. The small amount of BMC in group A in the absence of rhBMP-2 showed unsatisfied new bone formation, demonstrating slow repair rate. The relatively high BMC of group B was exciting at 4 weeks after surgery. And at 12 weeks significant differences of new bone mass were found between group A (70.10 ∼ 75.23 mg) and group B (78.23 ∼ 82.20 mg) (figure 5(b)). Moreover, it is clear that group C is superior to both groups A and B during the 12 weeks of the healing process. Although all defects showed substantial new bone formation, group C was closest to the normal control bone (figure 5(b)). Furthermore, the resulting BMD was similar to that of the BMC due to little individual differences of the radius area of the rabbits.
Figure 5. (a) The BMC of the three groups at the different stages of the bone reconstruction process. (b) The BMC and BMD of the three groups and normal control group at 12 weeks of healing process (*p < 0.05 as compared between three groups and +p < 0.05 compared to normal group).
Download figure:
Standard image3.7. Histological observations
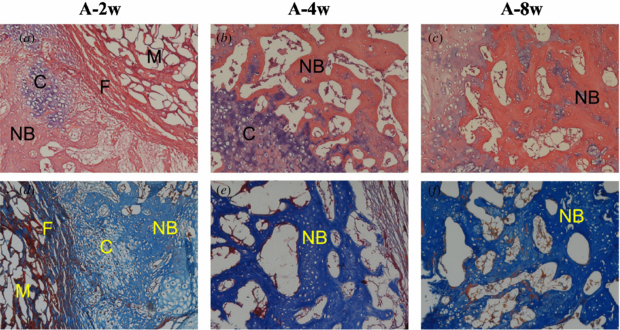
Group A (ACS alone). At 2 weeks, ACS implants were present in evidence and surrounded by fibrous connective tissue. On the outside of the fibrous tissue, newly formed cartilaginous tissue was easily observed. At 4 and 8 weeks, the ACS materials were almost degraded. Although new cartilaginous tissue and loose bone tissue could be seen, large vacancies consisting of fibrous and fatty elements were evident from the micrographs (figure 6).
Figure 6. Histological evaluation of group A at 2, 4 and 8 weeks after operation. NB: new bone, C: chondrocyte, M: materials and F: fibrous tissue (the top row shows HE stain, ×100; the bottom row shows Masson's trichrome stain, ×100).
Download figure:
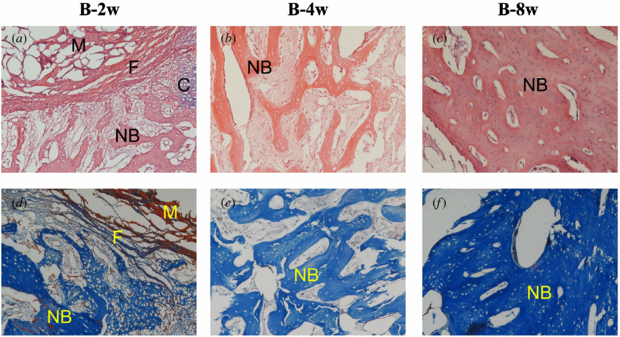
Standard imageGroup B (rhBMP-2/ACS implants). At 2 weeks, the paler pink new bone (figure 7(a)) was observed within loose fibrous connective tissue, and residual ACS was still visible without any significant adverse reaction. At 4 weeks, the newly formed bone consisted of woven bone and partially took over the material area. While lamellar bone was observed at 8 weeks, a number of voids still existed among the new bones.
Figure 7. Histological evaluation of group B at 2, 4 and 8 weeks after operation. NB: new bone, C: chondrocyte, M: materials and F: fibrous tissue (the top row shows HE stain, ×100; the bottom row shows Masson's trichrome stain, ×100).
Download figure:
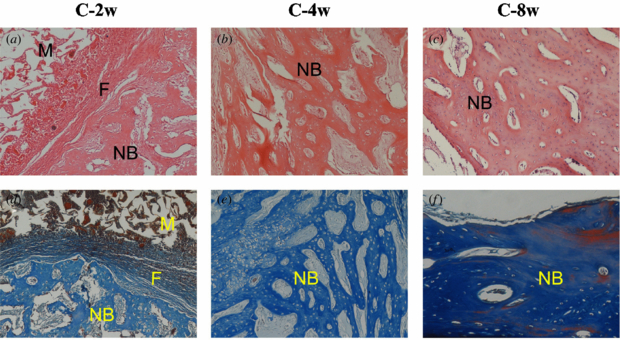
Standard imageGroup C (rhBMP-2/ACS+CMs implants). At 2 weeks, remnant CMs surrounded by ACS materials were present as red particles in the Masson stain images (figure 8(d)). In addition, less ACS materials and more newly formed bone with osteocytes were observed on two sides of the fibrous tissue. At 4 weeks, the quantity of new bone shown as woven bone seemed to be greater than that of group B. And the compact trabecular bone tended to be regular. The mature trabecular bone was stained dark blue with the Masson stain (figure 8(f)) and the lamellar bone seemed more compact at 8 weeks.
Figure 8. Histological evaluation of group C at 2, 4 and 8 weeks after operation. NB: new bone, M: materials and F: fibrous tissue (the top row shows HE stain, ×100; the bottom row shows Masson's trichrome stain, ×100).
Download figure:
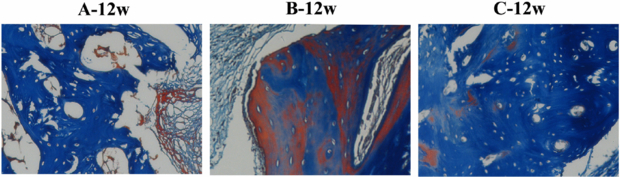
Standard imageFigure 9 shows the healing capacity of segmental bone defects after 12 weeks. Masson's trichrome stain samples were counterstained with aniline blue. The red color indicates that the trabecular bone was tending to mature, while the dark blue shows the mature bone.
Figure 9. Masson's trichrome stain of the three groups at 12 weeks after operation (×100).
Download figure:
Standard imageAlthough the neo-bone was observed from the Masson stain images, space with no bone formation still existed in groups A and B. Group C behaved relatively well in the healing of bone defects. The compact trabecular bone is arranged regularly.
3.8. Biomechanical testing
Three specimens from each of the three groups were used for the three-point bending test. Three intact specimens of right limbs were used as the normal control (normal group).
Figure 10 shows the flexural strength of the three types of implants at 12 weeks as compared with intact normal limbs. The maximum load for fracturing in the normal control bone was 246.41 ± 9.56 N, as compared to groups A, B and C which were 175.46 ± 5.44 N, 223.63 ± 8.98 N and 236.27 ± 10.57 N, respectively. No statistical differences were observed between group C and the normal control group, which suggests that the defect was almost healed, whereas the other implants (groups A B) showed significantly lower flexural strength than group C at 12 weeks.
Figure 10. Biomechanical stiffness of the regenerated bones as evaluated by the three-point bending test at 12 weeks post-implantation; the symbol (*) indicates significant difference in the maximum load as compared to group C (p < 0.05).
Download figure:
Standard image4. Discussion
In this study, rhBMP-2 was used to repair segmental bone defects because of its strong osteoinductive properties in bone regeneration. Many carriers, especially natural polymers, have been researched as delivery systems for the controlled release of BMP-2 [7–9, 21–23]. ACS approved by the FDA was used to load BMPs in this study, but the initial burst release of rhBMP-2 was not beneficial for bone healing (figure 2) [11]. For this reason, the development of a superior carrier material for sustained release is currently being investigated.
CMs were prepared to serve as the carrier of rhBMP-2 in this study. Previous studies have demonstrated that carriers equipped with adequate porosity allow higher absorbability [11]. The porous morphological structure of CMs was clearly observed from SEM images (figure 1(A)), which provided the possibility of using the CMs as a scaffold in bone engineering. The existence of the porous structure increased protein adsorption capacity [24]. The model protein absorption study showed that CMs with a polyporous structure could absorb more protein than those without a polyporous structure.
It is critical to consider that the carriers release the protein in a controlled way and maintain its bioactivity while releasing it. To avoid protein denaturation and degradation, a physical adsorption method was used to load rhBMP-2 onto the carriers [25, 26]. It is well known that extreme release profiles of BMPs such as long low-amount and initial burst are not beneficial to bone healing [11]. In order to confirm the optimal carrier of rhBMP-2, the release profiles of rhBMP-2 from three different carriers were investigated in vitro. Although the cumulative release profiles of the rhBMP-2/CMs group were superior to the rhBMP-2/ACS group, CMs alone cannot fill the large defected sites. Thus, ACS was used as an adjuvant to keep the CMs inside and prevent them from migrating away, which was closer to clinical application. Figure 2 showed that the rhBMP-2/ACS+CMs group exhibited minimal burst release followed by moderate release, while the rhBMP-2/ACS group resulted in a noticeable initial burst release. In view of the information mentioned above, the ACS/CMs carrier was the optimal carrier of rhBMP-2 in this study.
The results in vivo indicated that the implants containing rhBMP-2 did possess osteoinductive properties, and the rhBMP-2 releasing from the scaffolds still maintained its bioactivity. Figure 5(a) shows that the BMC of group A (without rhBMP-2) was lower than that of groups B and C during the repair process. At 8 and 12 weeks, the BMC of the rhBMP-2/ACS+CMs group (group C) continued to increase while the BMC of groups A and B kept a small growth trend, demonstrating that ACS+CMs scaffolds could retain the rhBMP-2 long enough at the defected site (figure 5(a)), thus, increasing retention of rhBMP-2 at the defect sites for a sufficient period to help the new bone formation [27]. As a result, group C exhibited bone bridging earlier as compared with groups A and B, almost completely healed the defects and presented recanalization of the bone marrow cavity (figures 3, 4), making the CMs/ACS scaffolds a promising candidate carrier of BMPs.
Natural polymers have been gradually considered promising candidates for tissue engineering because of their outstanding biocompatibility [28]. CS is renowned for its excellent biocompatibility with the human body environment [29]. In other research, CS is biocompatible to enable cell adhesion, proliferation and differentiation [17, 30], and ultimately supports in vivo bone regeneration [31]. In this work, CMs showed good tissue compatibility when rhBMP-2/ACS+CMs was implanted in defected sites in rabbits. At 2 and 4 weeks after the implantation, fibrous connective tissue was formed outside the materials without adverse reaction. Subsequently, the materials were degraded and almost replaced by newly formed bone. In groups A and B, ACS materials were surrounded by fibrous connective tissue in the early stage, and new bone was formed to fill the defects only partially, resulting in incomplete healing. The aforementioned results prove good tissue biocompatibility of the ACS and CMs composite scaffold, which could limit the effect of associated inflammation on bone healing [27].
From the histological observations of all groups, ACP remnants were present in evidence at 2 weeks and completely resorbed before 4 weeks. In group C, the CMs existed as red particles at 2 weeks (figure 8(d)), then degraded until the 8th week. The degradation of these materials matched the tissue in-growth at the defect site [32]. Moreover, histological observations indicated that neither the materials nor their degradation provoked inflammation or toxicity, corresponding with the need of scaffolds for tissue engineering [33].
Biomechanical testing of defected bone provides another method to evaluate the degree of healing of the defects. Biomechanical testing indicated that groups A and B exhibited significant difference in the maximum load for fracturing as compared to group C, while the maximum load of group C was close to the normal control bone (figure 10). Comparing these results, a strong correlation was found comparing histological and radiographic results with biomechanical flexural rigidity. Group C demonstrated the benefit of the addition of rhBMP-2 on CMs for improved bone regeneration.
5. Conclusions
The aim of this study was to prepare CMs/ACS composite scaffold close to clinical application as a carrier of rhBMP-2 and to investigate the osteoactivity of rhBMP-2 in the repair of large bone defects. The results showed that CMs were spherical in shape and had polyporous surface to absorb more protein, and a composite scaffold of CMs and ACS exhibited an ideal releasing profile. More importantly, the composite scaffold and rhBMP-2 could accelerate the repair of critical-sized radial defects, demonstrating great promise for the treatment of segmental bone defects.
Acknowledgments
The authors wish to express their gratitude for financial support from National Basic Research Program of China (973 Program, 2012CB933600), the State Key Program of National Natural Science of China (no 50732002), National Natural Science Foundation of China (nos 50973029 and 50873034), Program for Chang Jiang Scholars and Innovative Research Team (IRT 0825) and the scientific research foundation for the excellent young scholars from ECUST (no YD0157109). They also acknowledge the orthopaedic department of Xijing hospital-Fourth Military Medical University for the micro-CT technical assistance.