Abstract
Superparamagnetic iron oxide nanoparticles (SPIONs) and core-shell type nanoparticles, consisting of SPIONs coated with mesoporous silica and/or lipid, were synthesised and tested for their potential theranostic applications in drug delivery, magnetic hyperthermia and as a contrast agent. Transmission Electron Microscopy (TEM) confirmed the size of bare and coated SPIONs was in the range of 5–20 nm and 100–200 nm respectively. The superparamagnetic nature of all the prepared nanomaterials as indicated by Vibrating Sample Magnetometry (VSM) and their heating properties under an AC field confirm their potential for hyperthermia applications. Scanning Column Magnetometry (SCM) data showed that extrusion of bare-SPION (b-SPION) dispersions through a 100 nm polycarbonate membrane significantly improved the dispersion stability of the sample. No sedimentation was apparent after 18 h compared to a pre-extrusion estimate of 43% settled at the bottom of the tube over the same time. Lipid coating also enhanced dispersion stability. Transversal relaxation time (T2) measurements for the nanoparticles, using a bench-top relaxometer, displayed a significantly lower value of 46 ms, with a narrow relaxation time distribution, for lipid silica coated SPIONs (Lip-SiSPIONs) as compared to that of 1316 ms for the b-SPIONs. Entrapment efficiency of the anticancer drug, Doxorubicin (DOX) for Lip-SPIONs was observed to be 35% which increased to 58% for Lip-SiSPIONs. Moreover, initial in-vitro cytotoxicity studies against human breast adenocarcinoma, MCF-7 cells showed that % cell viability increased from 57% for bSPIONs to 82% for Lip-SPIONs and to 87% for Lip-SiSPIONs. This suggests that silica and lipid coatings improve the biocompatibility of bSPIONs significantly and enhance the suitability of these particles as drug carriers. Hence, the magnetic nanomaterials prepared in this work have potential theranostic properties as a drug carrier for hyperthermia cancer therapy and also offer enhancement of contrast agent efficacy and a route to a significant increase in dispersion stability.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Cancer is one of the most devastating diseases in the world, causing millions of fatalities every year. Over the past two decades, the mortality rate has significantly reduced due to considerable progress in diagnosis and treatment of the disease (Siegel et al 2018). However, all the current treatments such as surgery, chemotherapy and radiation are unpleasant, pose a risk of recurrence of disease and lack specificity, causing toxicity and undesirable side effects. To tackle the limitations of conventional anti-cancer treatments, a significant and increasing proportion of recent research has been focussed on understanding the biological interactions of existing drugs on a cellular level and developing new drugs (Fahs et al 2014, 2015, Palmer et al 2018) . Moreover, new strategies offering a targeted approach to deliver the drug to a cancer site, such as immunotherapy (Milling et al 2017, Riley et al 2019), gene therapy (Amreddy et al 2018), laser treatment (Doughty et al 2019) and hyperthermia (Sharma et al 2018, Blanco-andujar et al 2018, Phung et al 2019), have been explored.
Nanotechnology is emerging as a powerful tool in providing ways to manufacture materials to treat diseases including cancer. Nanoparticles in the size range between 1–100 nm have proven to be an effective therapeutic carrier system for drug delivery applications (Wakaskar 2018, Wang et al 2018, Aghebati-maleki et al 2020). Hydrophilic and/or hydrophobic drugs can be encapsulated in the nanoparticles, thus reducing the toxicity of the drug and enhancing its solubility. Moreover, their small size and large surface to volume ratio improve their blood circulation and thus enhance their intracellular uptake (Donahue et al 2019). As lipids are an integral part of a membrane, liposomes present an ideal biocompatible system and hence these are one of the oldest nanoparticles to be used in medicine (Patil-sen et al 2004, Alavi and Hamidi 2019, Lee and Im 2019). However, limitations associated with liposomes are in their pharmaceutical development i.e. reproducibility and reliability, long term structural stability and chemical instability or denaturation of the encapsulated compound in the manufacturing process (Sercombe et al 2015). Several other types of nanoparticles (NPs) have been extensively researched for therapeutic applications which include micelles, dendrimers, polymeric, metallic, silica and cell membrane coated nanoparticles (Narain et al 2017, Patil-sen et al 2019, Khan et al 2019, Janko et al 2019, Yang et al 2019).
Out of the mentioned NPs, superparamagnetic iron oxide nanoparticles (SPIONs) have shown great potential in theranostic applications, such as their use as a contrast agent in magnetic resonance imaging (MRI), in separation of biomolecules and in targeted drug delivery (Cardoso et al 2018, Patil-sen and Chhabria 2018, Janko et al 2019). As a consequence of their superparamagnetic properties, SPIONs are increasingly explored in hyperthermia cancer therapy (Hergt et al 2006, Verma et al 2014, Janko et al 2019). SPIONs exhibit no net magnetic moment in zero applied field but respond readily (relatively high permeability) in the presence of an external magnetic field and can thereby be directed specifically to the cancer cells. The therapeutic agent can then be released to the target cells by inducing local hyperthermia with an applied AC field which is advantageous for localized therapy.
Typically, MRI contrast agents are either paramagnetic lanthanide complexes, mostly containing gadolinium ions and which provide positive contrast in T1-weighted images (spin-lattice relaxation time), or iron oxide-based nanoparticles, such as SPIONs, which provide negative contrast in T2-weighted images (spin-spin relaxation time) (Estelrich et al 2015, Pellico et al 2019). The limitations associated with T1 contrast agents are poor detection sensitivity, short blood circulation and toxicity concerns (Estelrich et al 2015) whereas the major drawback of T2 agents is in its contrast mechanism, which produces a signal-decreasing effect (Pellico et al 2019). To overcome these limitations, there is a need to enhance the properties of current contrast agents or search for a dual or all-in-one contrast agent. Unlike the gadolinium-based ions, which only act as T1 contrasting agents, SPIONs can be tuned to function either as T1 or T2 by controlling the size of the NPs. SPIONs with particle size <10 nm, act as T1 agents whereas those above act as T2 (Estelrich et al 2015). Thus, controlling the size of SPIONs is a critical challenge for their application in MR imaging.
Another major issue associated with SPIONs is their propensity to aggregate (Hufschmid et al 2019). Agglomeration of nanoparticles further reduces their stability in dispersion which may have a negative effect on their in-vivo applications. One of the strategies that is researched for controlling their agglomeration is to coat the surface of SPIONs with materials such as polymers (Zhu et al 2018) and lipids (Luchini and Vitiello 2019). The coating may fully cover one individual particle or relatively small multi-cores rather than larger agglomerates of variable size and shape. Thus, such surface modification not only enhances the stability of the suspension but also aids in controlling the size of the NPs, both of which are important parameters for in-vivo applications of SPIONs in hyperthermia drug delivery and MRI applications (Hola et al 2015, Patil et al 2016).
Lipid coated SPIONs, also called magnetoliposomes, have gathered a lot of attention over the years and have been explored for their potential applications in biomedicine in a number of reviews (Soenen et al 2009, Reimhult 2015, Heidarli et al 2017). These hybrid systems provide synergistic properties as they combine the unique features of both i.e. properties of the magnetic NPs and biocompatibility of an outer lipid bilayer. Although these systems show great promise for hyperthermia cancer therapy, therapeutic efficacy of these systems, due to poor encapsulation efficiency of the drug, is still a challenge. In addition, there is a tendency in these articles to focus solely on either material characterisation or cellular efficacy as opposed to investigating both sets of properties as in the study reported here. Of those that consider both, Pradhan et al reported that thermosensitive folate-targeted DOX-containing magnetic liposomes with multifunctional properties showed enhanced in-vitro cytotoxicity against human epidermoid carcinoma and human cervical carcinoma in comparison with their non-magnetic folate-targeted liposome counterpart (Pradhan et al 2010). In another study, Kulshrestha and co-workers prepared paclitaxel loaded thermosensitive magnetoliposomes which were found to show a 46-fold higher drug release under an AC magnetic field of intensity 10 kA m−1 and a fixed frequency of 423 kHz (Kulshrestha et al 2012). They further reported that the in-vitro cellular cytotoxicity of the drug loaded magnetoliposomes against human cervical cells was much higher with magnetic hyperthermia than either chemotherapy or hyperthermia alone. Methotrexate modified thermosensitive magnetoliposomes were developed by Guo et al for precise cervical cancer therapy (Guo et al 2018). Light and magnetic hyperthermia triggered release of DOX increased its uptake into the tumour cells and enhanced its cytotoxicity to the cancerous cells as indicated by both in-vitro and in-vivo results. Szuplewska and researchers developed nanocarriers consisting of hydrophobic iron oxide NPs coated with the lipid bilayer for magnetic field-assisted selective delivery of DOX to breast cancer cells (Szuplewska et al 2019). Askari et al reported magnetoliposomes made of magnetic NPs and PolyEthylene Glycol-ated (PEGylated) liposomes for in-vitro DOX delivery against human breast cancer cells (Askari et al 2020). They compared their results with liposome-only PEGylated DOX and found the inclusion of a magnetic core enhanced drug encapsulation efficiency from around 25% to 37%.
Lipid coatings may also be applied to dual core-shell systems consisting of a magnetic NP core and non-magnetic shell and are defined here as nanocomposites or composite-magnetoliposomes. These systems offer the advantage of both drug encapsulation in the shell combined with the biocompatibility of the outer lipid layer when compared to just core-shell composites. Reports on these dual-coated systems in terms of their theranostic applications are limited. Pradhan et al developed a pH sensitive and thermosensitive system consisting of lipid-coated mesoporous silica-shell magnetic core nanoassemblies as a delivery system for thermochemotherapy of dual drugs i.e. DOX and paclitaxel (Pradhan et al 2014). They observed that the intracellular uptake and cellular toxicity, on human cervical cancer, breast cancer and human hepatocellular carcinoma, for the dual drug loaded system was higher than that for the single drug system which was further enhanced by magnetic hyperthermia. Sharifabad and authors produced lipid and/or mesoporous silica coated SPIONs for their potential application in delivery of Mitomycin C (Sharifabad et al 2015). They showed single and dual coatings resulted in very different drug loading and release profiles and thereby a possible route for tuning rate and load delivery to match that of clinical need. In another study, the researchers fabricated a system of soy-phosphatidyl choline/cholesterol lipid and/or mesoporous silica coated SPIONs, but in this case using DOX as the anticancer drug. They tested the cellular toxicity of these novel particles in-vitro against breast cancer and glioma cells and found that magnetic hyperthermia lowered the survival rate of these cells by around 20% compared to that of DOX alone (Sharifabad et al 2016).
To the best of our knowledge there are no reports on contrast agent investigations using a three-component composite-magnetolipsome. Herein, we report on the fabrication of such a system, consisting of a SPION-silica core-shell coated with an outer lipid layer, and on its potential and novel application in enhancing the T2 relaxation time parameter required for negative contrast in MRI imaging. In addition, we report on the increased stability provided by the dual coating using a fully quantitative technique (scanning column magnetometer) that takes advantage of the magnetic properties in order to provide complete settling profiles of particle concentration over the height of a column of dispersion. Enhancements of in-vitro drug-loading efficiency and release due to the silica coating and in biocompatibility due to the lipid layer are also studied. These are important findings for MRI and hyperthermia-mediated targeted drug delivery and supports their potential use as theranostic systems for cancer therapy.
2. Materials and methods
Iron (II) chloride tetrahydrate (FeCl2, 4H2O), Iron (III) chloride hexahydrate (FeCl3, 6H2O), cetyl trimethyl ammonium bromide (CTAB), tetraethyl orthosilicate (TEOS), dipalmitoyl phosphatidylcholine (DPPC), cholesterol (Ch), phosphate buffered saline (PBS), methanol, chloroform, ammonium hydroxide (NH4OH, 28–30 wt% ammonia in water), sulphuric acid (H2SO4, 0.1 M in water) were purchased from Sigma-Aldrich, UK. Doxorubicin hydrochloride (DOX) was purchased from Cayman Chemical. PrestoBlue cell viability reagent was obtained from Invitrogen. Eagle's Minimum Essential Medium (EMEM), Fetal Bovine Serum (FBS), L-glutamine, Non-Essential Amino Acids (NEAA), and Sodium Pyruvate were purchased from Lonza, UK. All the chemicals were analytical grade and were used as purchased. Non-cancerous human foetal glial cell line, SVG p12 and human breast cancer commercial cell line, MCF-7 (ATCC) were kindly provided by UCLan's Biomedical Research Facilities. Milli-Q water (resistivity = 18 MΩ·cm) was used in all experiments.
2.1. Synthesis of SPIONs
Bare SPIONs (bSPIONs) were prepared by the co-precipitation method using ferrous and ferric chloride, in 1:2 mole ratio in alkaline media and under an inert atmosphere, as previously reported (Majid et al 2017). Briefly, 8.46 g (0.04 mol) FeCl2, 4H2O and 22.95 g (0.08 mol) FeCl3, 6H2O were dissolved in 500 ml deionized water previously degassed with nitrogen. The mixture was kept at 80 °C under nitrogen atmosphere while 50 ml of aqueous NH4OH (28–30 wt%) was added drop wise to the mixture over 30 min under vigorous stirring. The reaction was allowed to proceed for a further 1 h under nitrogen atmosphere. The black reaction product, Fe3O4, was washed to neutral pH several times using deionized water and magnetic separation.
2.2. Synthesis of silica coated SPIONs
Mesoporous silica coated SPIONs (SiSPIONs) were prepared using a surfactant templating approach via a hydrolysis and condensation mechanism. 480 mg of bSPIONs were dispersed in 200 ml of methanol/water (4/1, V/V) solution. 14 ml of NH4OH (28–30 wt%) solution was added to the mixture and stirred for 5 min. 10 g CTAB was then added to the solution and the suspension was homogenized by an ultrasonic bath for 15 min. To the reaction mixture, 6 ml TEOS was added drop-wise under vigorous stirring for 16 h. The resultant black product was isolated from the reaction mixture and washed several times with acidic methanol solution (2/49/49 v% of 0.1 M H2SO4/methanol/deionized water) in an ultrasonic bath to remove the surfactant from the mesopores, followed by subsequent washing with deionized water.
2.3. Preparation of lipid coated bSPIONs/SiSPIONs (Lip-SPIONs/Lip-SiSPIONs)
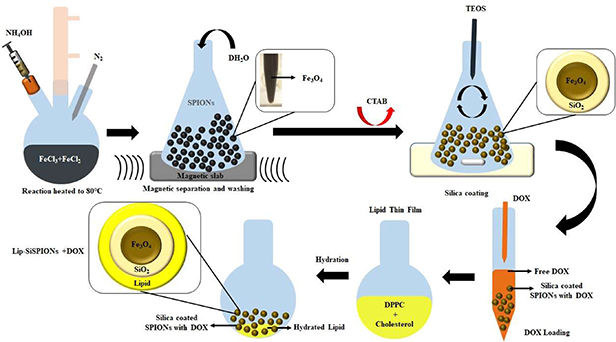
Lipid coated nanoparticles were prepared by the thin film hydration method. The lipid mixture of DPPC/Ch, in 7:3 (w/w ratio) was prepared by dissolving 42 mg of DPPC and 18 mg of Ch in 3 ml chloroform/methanol (2:1 v/v) in a round bottomed flask. The organic solvent was removed under vacuum by a rotary evaporator, using a water bath at 50 °C for 1 h. A thin film of lipid was formed inside the flask which was further dried under nitrogen gas for 2 h to remove any traces of organic solvent remaining. The lipid film was hydrated by adding 6 ml of deionized water, vortexed for 2 min, and then mixed using a rotavapor (without vacuum) in a water bath at 50 °C for 1 h and finally kept for annealing at room temperature for 2 h. 60 mg of bSPIONs in 6 ml deionized water was added to the lipid mixture to make a 1:1 Lip-SPION nanocomposite. The lipid-SPIONs nanocomposite was placed in an ice bath and ultrasonicated (ULT065 from Ultrawave, Cardiff, UK) for 5 min, with a continuous pulse at 40% power. The excess lipid in the suspension was removed using magnetic separation. Lipid coated SiSPIONs were prepared following the same procedure. Figure 1 summarizes the synthesis procedure for preparation of lipid and/or silica coated SPIONs.
Figure 1. Schematic representation of synthesis procedure for the SPION core and core-shell SPIONs.
Download figure:
Standard image High-resolution image2.4. DOX loading onto bSPIONs, SiSPIONs, and Lip-SPIONs and Lip-SiSPIONs
The prepared nanoparticles were dispersed for 15 min using a bath sonicator. DOX loaded nanoparticles were prepared by mixing a 1:500 (w/w) ratio of DOX: nanoparticle, at 18 °C using end over end rotation for 48 h. The amount of DOX that was loaded into the nanoparticles at different time intervals was determined by using a UV spectrophotometer. To quantify the amount of drug loaded into the nanoparticles, the drug loaded nanoparticles were centrifuged at 2000 rpm for 1 min followed by magnetic separation. The absorbance of the supernatant was measured at λ = 484 nm and was compared with a pre-established curve of known DOX concentrations in DMSO: water (1:1) to establish the concentration of the drug in the supernatant. The calibration curve is shown in figure A2 of the appendix section. The amount of drug loaded in the nanoparticles was obtained by measuring the difference in concentration of DOX between the amount initially added, [T(DOX)], and the amount remaining in the supernatant, [S(DOX)], over time. All the measurements were carried out in triplicate. Hence, the percentage of drug entrapment efficiency (% EE) was calculated using equation (1):
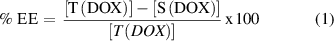
DOX-loaded nanoparticles were washed 3 times with deionized water and stored at 4 °C until further use for lipid coating, drug release studies and in-vitro cytotoxicity studies.
2.5. Characterisation
Magnetic measurements were performed at room temperature using an in-house 6 kOe Vibrating Sample Magnetometer (VSM). The samples were first dried and ground into a fine powder and then packed into plastic tubes of length ∼10 mm and internal diameter ∼1.9 mm. Typically 20–50 mg of magnetic materials were used for the measurements. The saturation magnetization values were expressed as (emu/g).
Transmission electron micrographs were obtained from a JEOL JEM2000EX (JEOL, Japan) instrument operating at an accelerating voltage of 200 kV. TEM samples were prepared by placing a drop of sample dispersion onto a carbon-coated copper grid using a pipette and drying at room temperature before placing into the TEM instrument. The images were collected using a digital camera and analysed using Gatan software.
The ability of the nanocomposites to generate heat in the presence of an oscillating magnetic field was evaluated by a DM100-Series Nanoheating Instrument (nanoScale Biomagnetics, Spain), at field frequency of 406 kHz and flux density of 200 gauss (0.02 T). The vial with dispersion containing magnetic nanoparticles at a concentration of 10 mg ml−1 was placed inside a magnetic coil loop which generated an oscillating magnetic field. The temperature was measured using a fibre optic sensor probe by dipping it in the dispersion. System embedded software, MaNIaC, was used to control the experimental parameters and to collect the generated data. The maximum temperature was set at 43 °C which is sufficient for classical hyperthermia treatment for cancer therapy (Kobayashi 2011). The efficiency of induced heat of magnetic materials was measured as the specific absorption rate (SAR). SAR reflects the heat transformation from magnetic energy, as shown in equation (2).
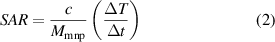
where c is specific heat capacity of the medium and  reflects the initial slope of temperature change as a function of time during the heat induction and Mmnp is the mass fraction of magnetic nanoparticles (Rovers 2010, Vallejo-fernandez et al 2013). The medium of dispersion was water in our samples and since the amount of magnetic nanoparticles was negligible compared to the amount of water, the value of c can be taken as the specific heat capacity of water = 4.184 J g−1 K (Motoyama et al 2008).
reflects the initial slope of temperature change as a function of time during the heat induction and Mmnp is the mass fraction of magnetic nanoparticles (Rovers 2010, Vallejo-fernandez et al 2013). The medium of dispersion was water in our samples and since the amount of magnetic nanoparticles was negligible compared to the amount of water, the value of c can be taken as the specific heat capacity of water = 4.184 J g−1 K (Motoyama et al 2008).
2.6. Stability measurements
A Scanning Column Magnetometry (SCM) technique was used to examine the effect of lipid coating on SPIONs on the dispersion stability of the nanoparticles. The effect of extrusion on dispersion stability of bSPIONs was also investigated. For this, the SPIONs were extruded 5 times through a 100 nm polycarbonate membrane using a lipofast extruder (Avestin Europe GmbH, Germany). Full details of the SCM technique can be found elsewhere (Mercer and Bissell 2013), but in summary this includes the nanoparticle dispersion being filled into a vertically held glass tube with internal diameter 11 mm to produce a column height of 10 to 12 cm. This is then placed in the otherwise empty core of a coil that forms part of an oscillatory circuit. By effectively scanning the coil over the height of the tube, any magnetic material present at that point causes an increase in the coil inductance and a corresponding shift in the resonance frequency, ΔF, from its sample-free value of 1 MHz. As this is directly proportional to the particle volume concentration, profiles of ΔF as a function of column height correspond to a whole concentration profile of the magnetic fluid. By obtaining a series of these profiles over time, any change in magnetic particle concentration due to sedimentation, and hence the dispersion stability, can be investigated.
2.7. T2 measurements
Relaxation times for protons were measured using a Bruker Minispec (mq 60) bench-top relaxometer operating at 60 MHz (magnetic flux density: 1.41 T). The transversal relaxation times (T2) were determined by the Carr-Purcell-Meiboom-Gill (CPMG) pulse sequence. The CPMG is a type of spin echo pulse sequence consisting of a 90° radio frequency (RF) pulse followed by an echo train induced by n successive 180° pulses (refocusing pulses): (90° − τ − {180° − 2τ}sn) (De Andrade et al 2011). T2 relaxation time distributions for all samples were computed by applying the CONTIN algorithm (Bruker Software) to the measured data. Data were fitted using a biexponential curve to detect multiple components (fast and slow T2) contributing to the relaxation. All measurements were carried out at 37 °C and repeated at least in triplicate.
2.8. DOX release efficiency of nanoparticles
The drug release studies were carried out in PBS. DOX loaded nanoparticles were washed three times and then incubated in PBS at 37 °C using end over end rotation for 48 h. Time dependent release profiles were obtained using UV absorbance at λ = 484 nm and by comparing the data with the pre-established curve of known DOX concentrations in PBS. The release of DOX from the nanoparticles using magnetic hyperthermia to raise the temperature to, and then hold at, 37 °C was also examined and compared with that in the absence of a magnetic field.
2.9. In-vitro cytotoxicity studies
The cytotoxicity of the nanoparticles was investigated against MCF-7 cells. The cells were cultured in EMEM supplemented with 10% (v/v) FBS, 1% NEAA and 1% L-glutamine in a humidified incubator at 37 °C, 5% CO2. Cells were harvested at the logarithmic growth phase and seeded in 96-well plates at a density of 2000 × 104 cells in 90 µl EMEM per well. Cells were maintained in complete growth medium for 24 h at 37 °C in a humidified atmosphere with 5% CO2. After 24 h, the medium was removed and replaced with 90 μl of media containing nanocomposites in different concentrations, i.e. 125, 250 and 500 μg ml−1 (which correspond to 0.25, 0.5 and 1 μg ml−1 DOX concentrations respectively). The dose dependent cytotoxicity was evaluated by incubating cells for 24, 48 and 72 h and performing a PrestoBlue assay. These concentrations of nanocomposites were chosen based on the initial dose dependent studies carried out on nanoparticles and DOX. The nanocomposites were also tested for their biocompatibility and cellular toxicity against in-house SVG p12 cells which are non-cancerous foetal glial cells.
To perform the assay, 10 μl of PrestoBlue reagent was added to each well. The plates were covered with aluminium foil and incubated for 30 min. The fluorescence was measured using excitation at 535 nm and emission at 612 nm using a Tecan Genius Pro Plate Reader. From these results, the % cell viability was calculated using equation (3)
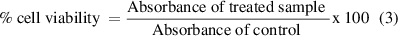
2.10. Statistical analysis
All results are expressed as the mean of three independent experiments at three different times with error bars presenting the standard deviation. For the cytotoxicity study, in each experiment, measurements were carried out in six wells. IC50 values were calculated using Microsoft Excel. A linear regression trendline was applied to the log-linear plots of cell viability as a function of concentration. The software's Data Analysis tool was employed to calculate the 95% CI values. Statistical analysis was also performed using Microsoft Excel, t-tests, one-tailed, paired: where p < 0.05 was considered statistically significant.
3. Results and discussion
3.1. VSM results
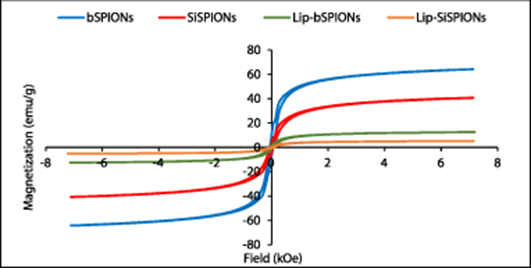
The magnetization curves for the prepared magnetic nanoparticles obtained from VSM measurements are presented in figure 2. In the bulk these iron oxides are ferrimagnets with distinct hysteresis loops and associated remanence and coercivity. For example, magnetite has a saturation magnetization, Ms, of around 92 emu g−1 and coercive field values, Hc, in the range 100 to 400 Oe (Ahmadzadeh et al 2018). However, reduction in size into the range of tens of nanometres leads to a change in magnetic behaviour: at room temperature the energy of thermal agitation is now greater than that of the magnetic ordering and the material exhibits a paramagnetic-like response, with zero magnetic moment in zero field (Dunlop 1973, Goya et al 2003, Gupta and Gupta 2005). On application of an external field the moments readily align to give relatively large Ms values compared to conventional paramagnetism and hence the term Superparamagnetic. For biomedical applications, such superparamagnetic particles are preferred due to this property of no retained magnetization (zero remanence, Mr) once the magnetic field is removed.
Figure 2. Magnetization curves for bSPIONs SiSPIONs, lipid coated bSPIONs and lipid coated SiSPIONs.
Download figure:
Standard image High-resolution imageAs can be seen from the figure 2, the prepared nanoparticles show small hysteresis with coercivity values of order tens of Oe, being indicative of the superparamagnetic nature of the synthesised particles (Goya et al 2003). It should also be noted that increasing dipolar interactions between particles results in the emergence of hysteresis in otherwise purely superparamagnetic particles (Petracic 2010). As there is evidence of agglomeration in the TEM micrographs of figure 3, this may be responsible in part for the results observed here. The saturation magnetization value for the bare SPIONs of 64 emu g−1 is lower than the bulk value of 92 emu g−1 as expected in particles of this size and is comparable with results reported elsewhere for the size range here of 5 to 20 nm (Kulshrestha et al 2012, Mascolo et al 2013). Moreover, it was observed that silica and/or lipid coating decreased the saturation magnetization of SPIONs further. The lower values due to the silica and lipid coatings are given in table 1 and are expected due to the dilution of the magnetic material content within the volume of the sample by the non-magnetic shells. The exact effect of surface coating on magnetic properties of SPIONs still remains unclear and different findings have been reported for different kinds of magnetic NPs and coating materials. However, reduction in saturation magnetization of Fe3O4 upon coating with silica and lipid has been reported by others (Rodrigues et al 2017, Morales et al 2019, Husain et al 2019). Husain et al and Morales and co-workers found that decrease in the saturation magnetization value of SPIONs was dependent on the thickness of the silica layer; thicker the silica layer lower is the saturation magnetization (Morales et al 2019, Husain et al 2019). We have previously observed a similar trend for the peptide coated SPIONs and SiSPIONs (Majid et al 2017).
Table 1. Saturation magnetization and coercivity values for the prepared nanoparticles.
| Nanoparticle | Saturation magnetization (Ms), emu g−1 | Coercivity (Hc), kOe |
|---|---|---|
| bSPIONs | 64 | 0.06 |
| SiSPIONs | 41 | 0.06 |
| Lip-bSPIONs | 13 | 0.06 |
| Lip-SiSPIONs | 5 | 0.04 |
Figure 3. TEM images for (a) bSPIONs; (b) SiSPIONs and (c) lipid-SiSPIONs.
Download figure:
Standard image High-resolution image3.2. TEM results
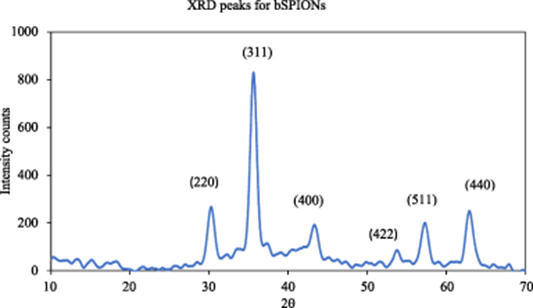
TEM images show that the prepared nanoparticles are spherical in shape (figure 3). The particles also show aggregation and exhibit polydispersity with size ranging between 5–20 nm for bSPIONs (figure 3(a)). Further, the bSPIONs were observed to be black in colour indicating that the prepared nanoparticles were predominantly magnetite i.e. Fe3O4, however, it may also contain a small percentage of brown coloured maghemite i.e. Fe2O3, as reported in the literature (Kim et al 2012, Sharifabad et al 2015). Both magnetite and maghemite would show superparamagnetism at this size and would show similar magnetic properties. The powder x-ray diffraction peaks for the synthesised bSPIONs are shown in figure A1 in the appendix section. Peaks corresponding to Miller indices 220, 311, 400, 422, 511 and 440 in the 2θ values between 30 to 70 were observed which are the fingerprint of magnetite Fe3O4 (Sun et al 1998). The micrograph for SiSPIONs indicates that the multiple SPION particles are coated within a mesoporous silica structure (figure 3(b)). SiSPIONs also are polydisperse with the average diameter of the particles ∼150 nm. The size of the particles further increased to ∼200 nm upon lipid coating (figure 3(c)).
3.3. Magnetic hyperthermia
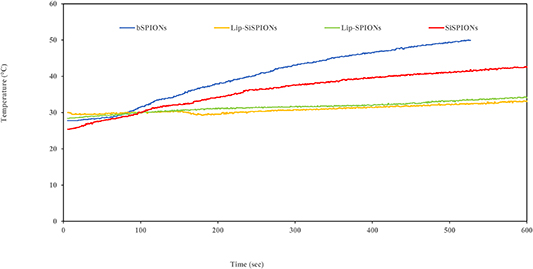
The results of magnetic field induced hyperthermia for the prepared nanoparticles as a function of time are presented in figure 4. bSPIONs are the most effective in generating heat in the presence of an AC magnetic field and the heat-generating ability decreased for silica and lipid coated nanomaterials. In the case of the bSPIONs dispersion, the temperature increased to above 43 °C within 5 min, whereas for the SiSPIONs dispersion, it took twice as much time to reach to that temperature. Both types of lipid-coated magnetic nanoparticles showed the slowest temperature increase and reached only around 32 °C in 10 min of exposure to the AC field and took approximately 45 min to reach 43 °C (only first 10 min shown).
Figure 4. Time dependent temperature rise curve for bSPIONs, SiSPION, Lip-bSPION and Lip-SiSPION when the particles were subjected to an oscillating magnetic field of frequency 406 kHz and magnetic induction of 200 gauss. (0,02 T)
Download figure:
Standard image High-resolution imageSAR values for the dispersion of bSPIONs with a concentration 10 mg ml−1 was calculated to be 22.4 W g−1. Theoretically, SAR depends on field parameters, strength and frequency as well as geometrical and magnetic properties of nanoparticles (Rosensweig 2002, Kumar and Mohammad 2011). Hence, although a true comparison can only be made using (i) a sample vessel of the same size, shape and material, (ii) same size, shape and compostion of core-shell SPIONs and (iii) the same dispersion volume concentration; it is interesting to note that similar SAR values for bSPIONs have been reported by others (Muller et al 2005, Zhang et al 2007, Kulshrestha et al 2012).
The SAR value for the lipid coated SPIONs was found to be 7.7 W g−1. The higher vlaue for the bSPIONs compared to the coated particles is not surprising if the heating mechanism is hysteresis-dominated as reported elsewhere (Vallejo-fernandez et al 2013, Vallejo-fernandez and O'grady 2013). Under AC field conditions, the narrow loops of the near-DC field curves of figure 2 would be open, with distinct remenance and coervivity more typical of that seen in the bulk ferrimagnetic material. As the heat generated is proportional to the total area of the hysteresis, it is clear that in the coated samples the dilution of magnetic volume fraction by the non-magnetic coating will reduce Ms values and hence the hysteresis area. For fully-open loops with the same saturation magnetisation of figure 2 we would expect the heating effect for bSPIONs > SiSPIONs > Lip-bSPIONS > Lip-SiSPIONS which is consistent with the measured heating effects shown in figure 4.
A Similar trend in SAR values has been reported by Kulshrestha et al for bSPIONs and magnetic liposomes with a decrease in the SAR value from 21.5 W g−1 for SPIONs to 14.2 W g−1 for magnetoliposomes (Kulshrestha et al 2012). However, Pradhan and co-workers found an opposite behaviour and an increase in SAR value of mesoporous silica coated magnetic NPs upon lipid coating. They suggested a possible reason could be that the lipid coating provided a better dispersion stability to the NPs which enhanced the movement of NPs and thus, contributed to the Brownian relaxation and heat generating ability. However, as previously mentioned the SAR depends on magnetic field parameters as well as on the properties of NPs, concentration etc, so the results cannot be truly compared.
3.4. SCM results
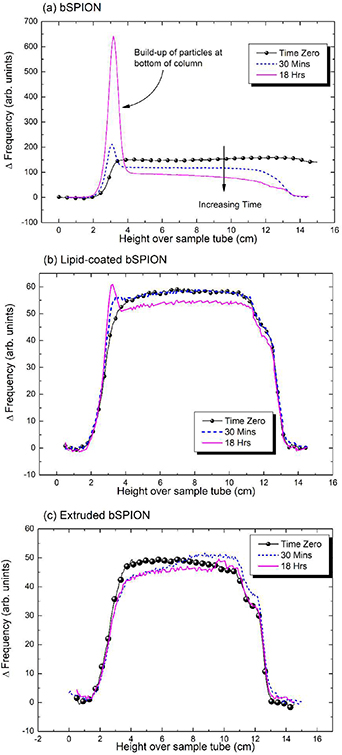
Preliminary studies on the dispersion stability of some of the prepared nanoparticles were conducted using an SCM. The results from SCM measurements for bSPIONs, extruded bSPIONs and Lip-SPIONs are shown in figure 5. As can be seen from figure 5(a), bSPIONs show poor dispersion stability. The peak in the concentration of particles at the bottom of the column shows sedimentation has occurred after only 30 min and by 18 h a significant number of particles are now in the lower layers. As the shift in frequency of the ordinate axis is directly proportional to the particle volume concentration and the diameter of the column is constant over its height, integration of the profile curves yields a value proportional to the total particle content. Hence, 43% of the particles by volume were estimated to have settled out after 18 h from the ratio of integration values from the bottom (at 2 cm) over the peak (out to 4 cm) compared to the value from over the whole of the column. The reduction in ΔF of the reasonably flat plateau as a function of time shown in figure 5(a) is indicative of Stokes'-like settling. Here, the particles settle in the same manner as a single particle in an infinite fluid, with any distribution in their fall velocities brought about by the distribution of particle diameters and with no evidence of the collective phenomena of hindered settling as observed in other magnetic suspensions (Mercer and Bissell 2013).
Figure 5. SCM profiles of (a) bSPION; (b) Lip-bSPION and (c) bSPION extruded 5 times through 100 nm membrane. There is a clear and significant increase in stability due to extrusion of the bSPION colloid, with no sedimentation apparent after 18 h in (c) compared to 43% by volume settled at the bottom of the tube in (a). Coating-alone also enhanced stability as shown in (b), where only 13% had settled over the same time period.
Download figure:
Standard image High-resolution imageThe Lip-SPIONs are more stable with only 13% estimated to have settled at the bottom after 18 h (figure 5(b)) indicating that the dispersion stability of bSPIONs was significantly improved upon coating with lipid. Moreover, the bSPION dispersion which was extruded 5 times through a 100 nm membrane showed no particle sedimentation after 18 h (figure 5(c)), unlike the un-extruded sample in which the particles started settling at the bottom of the SCM tube within 30 min. This is consistent with a significant increase in the number of monodisperse nanoparticles in the extruded sample whereas un-extruded bSPIONs showed polydispersity, as also seen from TEM (figure 3(a)). As colloidal stability is important for biological applications (Hufschmid et al 2019), this result warrants further investigation on other SPIONs, both coated and uncoated. Whilst it is generally known that extrusion either enhances the stability of NPs and/or aids improved monodispersity (Sabaté et al 2008, Bartenstein et al 2016), this is not quantified in terms of sedimentation over complete concentration profiles. Thus, we show that use of the SCM method provides a simple and convenient route to characterise and quantitatively measure this important parameter and to the best of our knowledge is reported here in these systems for the first time.
3.5. T2 results
The distribution of the T2 relaxation times is reported in figure 6 and single T2 values for all samples are reported in table 2. Because of the strong dependence of relaxivity from the amount of superparamagnetic material, all samples were measured considering the same iron oxide concentration at 1.5 mg ml−1. At this concentration, bSPIONs showed a single peak of T2 contribution at 1316 ms and this value is used as a standard for comparison with the other samples. Relaxation time analyses for Lip-SPIONs displayed two peaks at 253 ms and 34 ms while SiSPIONs showed three peaks at 307 ms, 78 ms and 19 ms. The presence of more than one peak can be explained considering the effect of nanoparticles' aggregation (clustering) and the presence of unreacted materials, such as residual lipids or silicon, since the measured relaxation times are very close to those of the same reagents in aqueous dispersion. In particular, the peaks at higher relaxations times can be attributed to the residual materials while peaks at lower relaxation time are related to a different state of aggregation, as confirmed by the large relaxation time distribution curves. Indeed, it is well-known that silica has the ability to influence the relaxation time distribution due to its hydrophilicity while the lipid contribution to relaxivity reduces the relaxation times of the free water molecules (Ananta et al 2010, Russo et al 2016, Vecchione et al 2017a, 2017b, De Sarno et al 2019).
Figure 6. T2 characteristic relaxation time distributions for the prepared particles.
Download figure:
Standard image High-resolution imageTable 2. T2 values for the prepared nanoparticles.
| Nanoparticle | T2 relaxation peaks (ms) | ||
|---|---|---|---|
| P1 | P2 | P3 | |
| SPIONs | 1316 ± 1 | ||
| Lip-SPIONs | 253 ± 5 | 34 ± 6 | |
| SiSPIONs | 307 ± 1 | 78 ± 1 | 19 ± 2 |
| Lip-SiSPIONs | 46 ± 1 | ||
However, a significant result in terms of T2 contribution is reported for the Lip-SiSPIONs; where a single and low T2 value at 46 ms with a very narrow relaxation time distribution curve is shown with respect to the bSPIONs at the same concentration. This result is extremely promising since the low T2 value is clinically relevant for the quality of MRI images (Hobson et al 2019) and also because the combination of the lipid and silica for the coating of the SPIONs is opening several biological opportunities on the uptake of safer SPIO- based nanoparticles. A slight shift is observed for the samples containing doxorubicin even though the same trend is also confirmed for all samples. Shortening of T2 relaxation time has been reported for SPIONs with surface attached gadolinium complexes and hybrid SPION-coordination polymer nanoparticles (Szpak et al 2014, Borges et al 2015). The dual-coated composite reported here may well provide a new class of SPION-based agents in terms of MRI contrast enhancement and further consolidates the suitability of our core-shell SPIONs as multi-functional nanoparticles.
3.6. Drug loading and release results
Both loading and release studies in an incubator were carried out at 37 °C up to 48 h and over the same set of time intervals as shown in figures 7(a) and (b) respectively.
Figure 7. (a) % entrapment efficiency of DOX in nanocomposites as a function of time; (b) DOX release from nanocomposites at 37 C as a function of time and (c) comparison of DOX release at 37 C in the absence and presence of AC magnetic field (n = 3; mean ±SD).
Download figure:
Standard image High-resolution imageThe amount of DOX that was entrapped into the nanoparticles at different time intervals was determined by using UV spectrophotometer and from the calibration curves for DOX obtained using prepared known concentrations (figure A2). The linear regression fit shown with an R2 value >99.85% gives confidence in the conversion of measured absorbance to DOX concentration. The entrapment efficiency for Lip-SPIONs after 48 h was found to be ∼35% whereas that for Lip-SiSPIONs it was ∼58% (figure 7(a)). The higher loading in SiSPIONs could be attributed to the mesoporous structure of silica which reduces drug leakage from the nanoparticles and hence shows better entrapment of the drug. There was no significant loading between 24–48 h, whereas maximum drug release was observed after 48 h (80%–90%).
The release of drug from Lip-SPIONs + DOX and Lip-SiSPIONs + DOX after 48 h of incubation was found to be ∼80%–85% (figure 7(b)). For comparison with release at the same temperature, but this time achieved using an AC magnetic field as shown in part 2 of figure 7(c), the incubator release at 48 h is shown again in Part 1 of the same figure. In the case of the AC field, it took 15 min to raise the temperature from RT to 37 °C; at which point the sample was removed before carrying out the release measurement. Remarkably, the release profiles after this short time are somewhere between those seen at 12 and 24 h in the incubator. As the heating mechanisms of the magnetic AC field method involves physical stirring (Brownian rotation), Néel susceptibility and hysteresis losses (Vallejo-fernandez et al 2013) this is very different from the near-static maintaining of a temperature in an incubator once it is in equilibrium and warrants further investigation.
For Lip-SPIONs 80% drug release was observed whereas for Lip- SiSPIONs, the drug release was seen to be much less, ∼57%. One possible reason for this may be that the porous silica structure results in better entrapment of the drug compared to lipid-coated and thereby hinders drug release. Thus, although drug loading is enhanced due to mesoporous silica, the drug release is decreased.
3.7. In-vitro cytotoxicity results
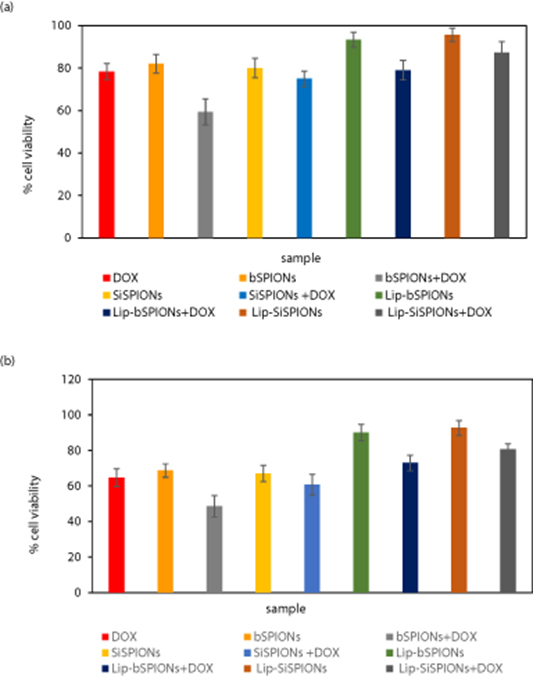
The cytotoxicity effect of nanoparticles at 37 °C and after 72 h is shown in figure 8 (particle concentration = 500 μg ml−1), against MCF-7 cancer cell lines. Please note, the DOX-only column is at a concentration of 1 μg ml−1 as this was the value of the DOX solution used for loading the NPs with this drug. Further details are given in the Materials and Methods section. The results are shown as percentage cell viability compared to untreated cells.
Figure 8. In vitro cytotoxicity effect of DOX (1 μg ml−1), and nanoparticles (with and without DOX) (500 μg ml−1 nanoparticle, which contain 1 μg ml−1 DOX), in MCF-7 after 72 h; n = 3, mean ±SD, t-test, one-tailed, paired. The ends of the horizontal bars above the columns indicate a pair chosen which is statistically significantly different. The colours indicate the different levels of significance. Black with values of *p < 0.05, green with **p < 0.01 and red with ***p < 0.001.
Download figure:
Standard image High-resolution imageAt this concentration, the cytotoxic effect of nanocomposites for incubation periods of 24 and 48 h was less prominent and the cell viability for the dual-coated nanocomposites was more than 90%. Hence, the results are only presented for an incubation time of 72 h. Other lower concentrations at 72 h, of 125 and 250 μg ml−1, resulted in values >73% for the two lipid-coated DOX-loaded systems (shown in figure A3 in the appendix). As systems above 70% are considered to be non-toxic (Szuplewska et al 2019) this prevented a comparison as a function of concentration across all the systems studied here. However, it can be noted in figure A3 that there is an overall trend across the samples in terms of lower concentrations resulting in higher viability. Whilst this is not conclusive, it can be considered (with caution) as indicative in terms of consistency within these results.
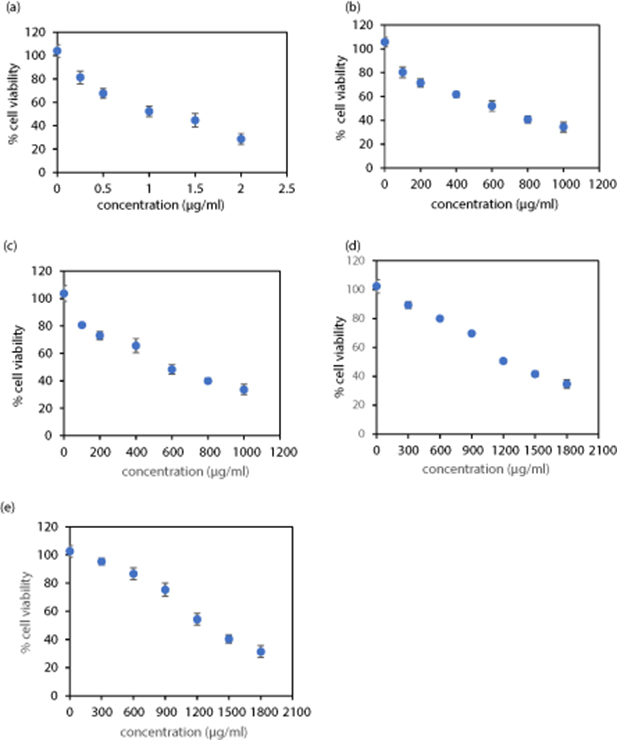
From our dose-dependent studies it was observed that bSPIONs induce toxicity at a concentration of 250 μg ml−1 and the cell viability was observed to be <70% (results shown in figure A4 in the appendix). This value is needed to determine an initial minimum concentration level at the start of an investigation. Similar results were reported previously (Sharifabad et al 2016). At the concentration value then determined and required for the coated systems reported here (of 500 μg ml−1), the bSPION and SiSPIONs viability are both approximately 57% and so are of similar toxicity to the viability value of 53% for DOX-only (1 μg ml−1), albeit a little less.
For lipid-coated nanoparticles, i.e. Lip-bSPIONs and Lip-SiSPIONs the cell viability was 82% and 87% respectively. Hence, as this is greater than 70% it is considered to be biocompatible. (Szuplewska et al 2019). Thus, a clear decrease in toxicity of the nanoparticles was seen for Lip-bSPIONs and Lip-SiSPIONs. This yields statistically significant levels of p values, that are all <0.05, when compared to their uncoated counterparts (bSPIONs and SiSPIONs) and is indicated by the bars in figure 8. Moreover, comparison here of our two lipid coated NPs with DOX-only, resulted in an even lower value of p < 0.001.
Overall, these results indicate that the toxicity of bSPIONs is significantly reduced (p < 0.01) upon dual coating the nanoparticles with silica and lipid.
For the DOX-loaded lipid-coated NPs, the silica shell of the dual coated bSPIONS resulted in a significant increase in cell viability from 55% to 73% (Lip-bSPIONs + DOX compared to Lip-SiSPIONs + DOX) as shown by the bar for the pair in figure 8. This decrease in cytotoxicity may be as a result of drug entrapment in the mesoporous structures of the silica coating that leads to a slower release of DOX. A similar trend was seen for the toxicity of DOX-loaded NPs without lipid coatings (bSPIONs + DOX compared to SiSPIONs + DOX), resulting in an increase in cell viability from 36% to 50%. However, in this case the pair are not significantly different.
It is interesting to note that the DOX loaded bSPION is more toxic than DOX or bSPION alone; although this should be treated with caution as they cannot be classified as being significantly different. It may be possible that physical adsorption between the DOX and bSPION molecules is such that the drug is released readily to the cells, but this would require detailed and further investigation.
DOX (1 μg ml−1), and nanoparticles without DOX, i.e. bSPIONs, SiSPIONs, Lip-SPIONs and Lip-SiSPIONs (500 μg ml−1) were used as controls. IC50 corresponds to the concentration of DOX or nanoparticle required to inhibit the growth of cells to 50% compared to the untreated cells. IC50 values were determined from the data shown in the dose-dependent cytotoxicity curves (figure A4 in the appendix) and the results are summarised in table 3. The IC50 value calculated for DOX-only of 1.1 μg ml−1 is within the range of 0.68–1.1 µg ml−1 (Fang et al 2014, Sharifabad et al 2016) as previously reported. Upon lipid coating, the IC50 values for bSPIONs and SiSPIONs increased which suggests that the lipid layer in both the Lip-bSPIONs and Lip-SiSPIONs reduces the toxicity and enhances the biocompatibility of the nanoparticles.
Table 3. Calculated IC50 and 95% CI for the control samples after 72 h.
| Test sample | IC50 (µg ml−1) (95% CI) |
|---|---|
| DOX | 1.13 (1.06–1.19) |
| bSPIONs | 610 (566–653) |
| SiSPIONs | 579 (552–623) |
| Lip-bSPIONs | 1244 (1114–1374) |
| Lip-SiSPIONs | 1302 (1166–1438) |
In addition, the cell viabilities for all of the nanocomposite systems and at all concentrations was tested against non-cancerous SVG p12 cells. In all cases, including exposure for 24, 48 and 72 h, the viability was observed to be above 75% (data not shown). Whilst this suggests that the NPs are biocompatible and non-toxic towards the non-malignant cells, this should now be tested further using non-cancerous breast cells such as MCF-10A. In this case it suggests a higher selectivity of NPs to cancer cells compared to healthy cells. Such selectivity of magnetoliposomes to cancer cells over the non-malignant cells has been previously reported by others (Rodrigues et al 2017, Szuplewska et al 2019). The selectivity of nanocomposites towards MCF-7 over SVG p12 cells may also be due to differences between morphology, permeability and metabolism of normal and cancer cells and increased sensitivity of MCF-7 cells to DOX.
Overall, the results at 37 °C clearly show that the best biocompatibility results are obtained from lipid-coatings and the best cytotoxicity is found in systems with silica coatings. Thus, the dual-coated lipid-silica magnetic nanocomposite system (Lip-SiSPION) prepared in this work offers the best compromise and potentially optimal in-vitro model (MCF-7 and SVGp12) for drug delivery and cytotoxicity. This promising result encourages further investigations to be carried out under hyperthermic conditions at 42 °C.
4. Conclusions
Composite drug carrier systems comprising of core SPIONs coated with silica and/or lipids have been developed with promising theranostic properties. To our knowledge, systems containing dual coatings of silica and lipid have not been studied for MRI applications as contrast agents and are reported here for the first time. In the prepared systems, the ultra-small size and superparamagnetic characteristics of SPIONs were retained in the silica and lipid coated SPIONs. An initial study on the effects of extrusion on the dispersion stability of bare SPIONs resulted in a significant improvement. Characterisation using an SCM technique provided a way of quantifying this important parameter in these bio-compatible magnetic NPs. As coated systems are intrinsically more stable than their uncoated SPION cores, such as the Lip-SPION system observed here, the extension of this extrusion methodology and SCM characterisation to such systems is the subject of further work. The ability to generate heat in the presence of an oscillating magnetic field and the favourable drug loading and release profile of the Lip-SiSPIONs show the suitability of these nanoparticles for hyperthermia drug delivery applications. MRI results indicate a low T2 value and very narrow relaxation time distribution curve for Lip-SiSPIONs. As this is clinically important for the quality of MRI images, these dual-coated composites may provide a new class of nanomaterials that enhances their effectiveness as contrast agents and warrants further investigation. In addition, lipid-coated nanoparticles (without DOX) have shown excellent biocompatibility in-vitro, against breast cancer cell line MCF-7 and non-cancerous foetal glial cells, SVG p12. In a preliminary investigation, DOX loaded nanocomposites showed high efficiency and selectivity as drug carriers against MCF-7 cell lines as compared to SVG p12. However, these results are only indicative and further studies are required before any definitive conclusions can be drawn.
The combination of lipid and silica for the dual coating of SPIONs is opening several biological opportunities on the uptake of safer SPION-based nanoparticles and hence these multifunctional nanoparticles can provide potential theranostic systems for diagnostic and targeted drug delivery in hyperthermia-mediated cancer therapy.
Funding
This work was carried out as part of Daphne Jackson Trust (DJT) fellowship which was jointly funded by the Royal Society of Chemistry (RSC) and the University of Central Lancashire (UCLan). YPS is grateful to the DJT, the RSC and UCLan for sponsoring the research.
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Appendix
Appendix. X-ray Diffraction
Powder x-ray diffraction pattern for bSPIONs is shown in figure A1. Peaks corresponding to Fe3O4 were observed.
Figure A1. XRD pattern for bSPIONs.
Download figure:
Standard image High-resolution imageAppendix. DOX calibration curve
A calibration curve was obtained by measuring absorbance of DOX solutions of various concentrations at a wavelength of 484 nm using a UV spectrophotometer and is presented in figure A2. Serial dilutions of DOX were made in water using 100 μg ml−1 stock prepared in 1:1 DMSO:water. From the calibration data points shown, a good linear fit was obtained with an R2 value >99.85%.
Figure A2. Calibration curve for DOX concentrations, 5, 10, 20, 40, 60 and 80 μg ml−1.
Download figure:
Standard image High-resolution imageAppendix. Cellar toxicity at lower concentrations
Cellular toxicity of DOX and the nanoparticles/nanocomposites on MCF-7 after 72 h at lower concentrations of DOX (0.25 and 0.5 μg ml−1) and NPs (125 and 250 μg ml−1) compared to DOX (1 μg ml−1) and NPs (500 μg ml−1) of figure 8 in the Results section are presented in figure A3.
Figure A3. In-vitro cytotoxicity of the nanoparticles and DOX on MCF-7 after 72 h; n = 3, mean ±SD. (a) 125 μg ml−1 of NPs with DOX concentration of 0.25 μg ml−1 and (b) 250 μg ml−1 NPs with DOX concentration of 0.5 μg ml−1. Overall there is higher viability across these profiles at lower concentration and so whilst not conclusive, is at least indicative of consistency within these results.
Download figure:
Standard image High-resolution imageAppendix. Dose-dependent cell viability
Dose-dependent cell cytotoxicity of DOX on its own and the control NPs (without DOX), on MCF-7 cells is presented in figure A4.
Figure A4. Dose-dependent cell cytotoxicity of (a) DOX; (b) bSPIONs; (c) SiSPIONs; (d) Lip-bSPIONs and (e) Lip-SiSPIONs on MCF-7 after 72 h; n = 3, mean ±SD. IC50 values were determined from log-linear plots of the same as detailed in the Materials and Methods section.
Download figure:
Standard image High-resolution image