Abstract
The inevitable formation of a protein corona upon contact of nanoparticles with different biological fluids is of great interest in the context of biomedical applications. It is well established that the surface chemistry of the respective nanomaterial has tremendous impact on protein adsorption, both in terms of the actual amount as well as the type of proteins adsorbed. In that regard, especially polyzwitterions are discussed as coating materials as they are known to partially inhibit protein adsorption. We herein present comparative incubation studies on iron oxide nanoparticles (either single core (SPION) or multicore nanoparticles (MCNP)) after coating with either polyanionic or polyzwitterionic polymeric shells based on polydehydroalanine (PDha). Apart from varying surface charge and chemistry, also the influence of incubation time and temperature on the formation and composition of a protein corona upon exposure to fetal calf serum was investigated. The amounts of adsorbed biomolecules were determined using thermogravimetric analysis. SDS-PAGE experiments revealed information on protein composition as major components of the biomolecule corona. Our results show that distinctly lower amounts of proteins are adsorbed onto polyzwitterionic hybrid nanoparticles in general, but also the corona composition varies as indicated by elevated relative ratios of medium molecular weight proteins (i.e. proteins 25–100 kDa) estimated by non-specific silver protein staining. In addition, increasing relative amounts of albumin (67 kDa) via specific Western blot assays on PDha-coated MCNP are detected.
Export citation and abstract BibTeX RIS
Introduction
During the last decades, magnetic nanoparticles (MNP) have gained huge interest in different research fields [1–4]. Especially for biomedical applications like hyperthermia [5–7] and drug delivery [8–10], such materials are of great interest as they allow mechanical manipulation or heating by external alternating magnetic fields. Additionally, they provide the ability of enhancing contrast in magnetic resonance imaging (MRI) [11, 12], and therefore have potential in simultaneous imaging and therapeutic approaches (theranostics). Regarding biomedical applications, potential nanoparticle systems face several problems: at first, the materials need to be inherently biocompatible and therefore, iron oxide nanoparticles are often preferred compared to nanoparticles made of alternative magnetic materials like Ni or Co [13–15]. Promising magnetic properties with regard to medical applications are observed for so-called multicore magnetic nanoparticles (MCNP) [16]. MCNP show superparamagnetic behavior in the absence, but ferrimagnetic behavior upon exposure to an external magnetic field. In combination with a superior heating performance, MCNP have high potential for hyperthermia applications [17–19]. In addition, SPIONs are of interest for medical applications, especially for applications where small hydrodynamic diameters and high aspect ratios are of interest as for example in the design of contrast agents for MRI [20–25].
The behavior of magnetic nanoparticles in a physiological environment was recently assessed [26]. As stated by the authors of this study, there are a large gap and limited understanding of nanoparticles in physiological systems. Independent of the nanoparticle nature, size, or shape, upon addition of such materials to a biological fluid biomolecule adsorption to the surface occurs [27]. Especially proteins are involved in that process and a so-called protein corona is formed [28]. This corona has tremendous influence on the dispersion stability of the particles in terms of secondary aggregation, recognition by the immune system, as well as nanoparticle cell interactions [29]. It has been shown that the formation of the protein corona is a dynamic process [30, 31], which is influenced by several factors, including medium composition [32], temperature and incubation time, as well as size and surface chemistry of the respective nanoparticles [33–36]. Besides effects on dispersion stability [37], it has also been shown that the protein corona plays an important role during cellular uptake [38] and subsequent intracellular processes once such materials have been internalized by cells [39]. Even more, the presence of a protein layer was shown to reduce unspecific cellular uptake for nanoparticles featuring a surface coating of poly(ethylene oxide) or poly(ethyl ethylene phosphate)—both belonging to the class of so-called 'stealth' polymers [40]. While several studies so far have described amount and composition [41], recent efforts have started to focus on a more quantitative description of the underlying kinetics governing protein corona formation [42] or, as an additional tool, measures to visualize the protein layer by electron microscopy—thereby also distinguishing between the 'hard' and the 'soft' part of the corona [43].
The surface chemistry of MNP can easily be tuned using different coating materials such as surfactants or polyelectrolytes and hybrid materials consisting of magnetic cores and an organic shell are widely used in biomedical research [1, 44, 45]. Polyelectrolyte coatings can stabilize NP dispersions due to high charge densities and, in addition, polymer platforms can be used to impart further functionality e.g. targeting, stealth [46, 47], or anti-fouling properties. Polyzwitterions, a subclass of polyelectrolytes bearing both cationic and anionic charges at every repeating unit of the polymer backbone [48, 49], often show anti-fouling properties in terms of reduced unspecific protein adsorption [50, 51]. This is dedicated to the high hydration capacity of the charged polymer backbones [52]. Typical examples for polyzwitterions showing anti-fouling behavior are poly(betaines) like poly(carboxybetaine acrylamide) [53], poly(sulfobetaine methacrylate) [54], or poly(carboxybetaine methacrylate) (PCBMA) [55]. The immobilization of PCBMA on the surface of magnetic nanoparticles has been shown to lead to reduced protein adsorption [56], and Pombo-Garcia et al reported similar effects for SPIONs after coating with 3-(dimethylamino)propylamine functionalized poly(maleic anhydride-alt-1-decene) [57].
We previously reported on the coating of superparamagnetic iron oxide nanoparticles (SPION) [58] or multicore magnetic nanoparticles (MCNP) [59] with polyelectrolytes based on polydehydroalanine (PDha), and also that the resulting hybrid materials are in general biocompatible [58]. Furthermore, as PDha shows varying charge in dependence of the solution pH, we could reversibly adsorb and desorb oppositely charged polyelectrolytes, proteins, or methylene blue as model cargo from PDha@MCNP hybrid nanoparticles by changing the pH of the dispersant [59, 60]. In the present study, we use both polyanionic poly(tert-butoxycarbonylamino acrylic acid) (PtBAA) and polyzwitterionic PDha for the coating of different magnetic nanoparticles, SPION and MCNP. Since both organic materials feature an identical polymeric backbone and molecular characteristics such as molar mass and dispersity, we used this to investigate the influence of negative- and zwitterionic charges and charge density on protein adsorption under varying conditions, and compared the results to those of pristine iron oxide nanoparticles. PDha is a charge tunable polymer which can undergo a change from a polycation at low pH to a polyanion at high pH. Around the isoelectric point (ca. 6.5) it shows polyzwitterionic behavior. By definition, this material is neither a true polyzwitterion nor a polyampholyte. As both charges are placed within the same repeat unit, the term polyzwitterion is used in the here presented study. Under the given experimental conditions (pH > pH for isoelectric point), the material features a negative net charge since negative charges dominate.
Both the pristine as well as the coated nanoparticle samples were incubated in fetal calf serum (FCS) for different times and at different temperatures. The resulting nanoparticles were then characterized concerning the amount of adsorbed biomolecules by means of zeta potential measurements, thermogravimetric analysis (TGA), and regarding qualitative protein composition of the biomolecule corona using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) with subsequent silver protein stain or Western blot assays. Corona-induced cytotoxic effects were investigated by cell viability testing using human brain microvascular endothelial cells (HBMEC) and PrestoBlue™ cell viability assays. Proteins form the vast majority of the corona; therefore we use the term protein corona.
Results and discussion
Nanoparticle synthesis and coating
Two types of iron oxide nanoparticles were used for the coating and incubation studies presented in this work: SPION with a diameter of 10 nm and raspberry-like multicore nanoparticles (MCNP), stable clusters of about 50 nm consisting of smaller nanoparticles of about 10 nm. These two types of nanoparticles represent commonly used model systems in medical applications and thus were chosen to perform our study with application-relevant core materials. Both particle types were prepared by co-precipitation from a mixed FeCl2 and FeCl3 solution by addition of a NaHCO3 solution, resulting in biocompatible nanoparticles consisting of solid solutions or a mix of maghemite (γ-Fe2O3) and magnetite (Fe3O4) with a predominant maghemite proportion [61, 62].
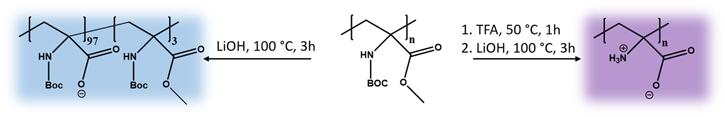
The coating materials PtBAA and PDha were obtained by deprotection of poly(tert-butoxycarbonylamino methacrylate) (PtBAMA) as described earlier [63]. The molar mass of the initial PtBAMA was determined as 22 800 g mol−1 with a dispersity of Ð = 2.94 using size exclusion chromatography (SEC). PtBAA was obtained by alkaline cleavage of the methyl ester, whereas PDha requires a two-step process—first, acidic deprotection of the amine using trifluoroacetic acid (TFA), followed by alkaline hydrolysis of the methyl ester (scheme
Scheme 1. Deprotection of PtBAMA to polyanionic PtBAA or polyzwitterionic PDha.
Download figure:
Standard image High-resolution imageThe resulting polyelectrolytes were used for SPION and MCNP coating as reported previously [59]. Briefly, the corresponding polyelectrolyte was dissolved in 0.01 M NaOH and titrated to pH = 5 using 0.01 M HCl. Both types of MNP were treated with ultrasound for 10 min and magnetically separated afterwards. Subsequently, the respective MNP were dispersed in the polyelectrolyte solution at constant weight ratios (MNP:polyelectrolyte = 1:8) and the mixture was ultrasonicated for 1 h, magnetically separated and washed with aqua bidest. (five times, scheme
Scheme 2. Schematic representation of the nanoparticle coating and incubation process as well as resulting protein contents.
Download figure:
Standard image High-resolution imageTable 1. Hydrodynamic radii determined by DLS, zeta potentials, polyelectrolyte (PE) contents, and calculated shell thicknesses of differently coated MCNP and SPION samples.
| Sample | Rh,N,appa [nm] | Zeta potentialb [mV] | PE contentc [%] | Calc. shell thicknessd [nm] |
|---|---|---|---|---|
| MCNP | 38 | +42 ± 7 | — | — |
| PtBAA@MCNP | 62 | −40 ± 12 | 4.0 | 3.3 |
| PDha@MCNP | 44 | −37 ± 4 | 5.0 | 2.1 |
| SPION | 5 | +33 ± 5 | — | — |
| PtBAA@SPION | 5 | −40 ± 5 | 2.0 | 0.2 |
| PDha@SPION | 3.5 | −43 ± 6 | 12.5 | 0.7 |
aDetermined via DLS, bDetermined using a Malvern ZetaSizer Nano ZS. cCalculated from TGA, dCalculated using equation (S1).
Compared to the pristine particles, all coated MCNP showed increased hydrodynamic radii in dynamic light scattering (DLS) measurements, decreased zeta potential, and increased weight losses in TGA, resulting in weight fractions of organic material of 4.0% (PtBAA@MCNP) and 5.0% (PDha@MCNP). All data is also summarized in tables S3–S8 in the supporting information.
Shell stability
To investigate the stability of the organic shell (PtBAA or PDha) under the given incubation conditions, both PtBAA@MCNP and PDha@MCNP were dispersed in water at a concentration of 4.3 g l−1 (the concentration used in the subsequent incubation studies) and heated to 70 °C for 20 min. Please note that the PDha@MCNP batch used for the shell stability investigations slightly varies from the one used for incubation experiments as here 7.5 wt% of PDha were adsorbed according to TGA data. Figure S5(A) shows the thermograms of PtBAA@MCNP and figure S5(B) those of PDha@MCNP, each before and after being stored at 70 °C for 20 min. On average, both the PtBAA (4.0% (before) and 4.5% (afterwards)) and the PDha content (7.5% (before) and 7.0% (afterwards)) are not significantly affected by the incubation procedure, at least within the error of the TGA method (table S1).
Incubation studies
For the formation of a protein corona at the surface of either PtBAA@MCNP or PDha@MCNP, the nanoparticles have been incubated in FCS serving as natural protein source. In brief, 750 μl of the respective particle dispersion with a concentration of 10 g l−1 were dispersed in 1 ml of preheated FCS and incubated in a heated water bath for 1, 5, 10 and 20 min and at temperatures of 25 °C, 37 °C, 50 °C and 70 °C. During incubation time the particles were treated with ultrasound (table S2). Afterwards, the samples were four times magnetically separated and washed with 1 ml of distilled water.
Pristine MCNP
In the following, only calculated protein contents will be shown, and both DLS as well as zeta potential results will only be briefly discussed. Figure 1 exemplarily shows the results obtained for pristine MCNP before and after incubation in FCS at 70 °C. The TEM micrograph in figure 1(D) shows an aggregate of pristine MCNP after 5 min incubation at 70 °C. The protein corona can be seen as gray shell around the iron oxide cores.
Figure 1. (A) Hydrodynamic radii and (B) zeta potentials of pristine MCNP before and after incubation at 70 °C for different times (for details see table S3), (C) thermograms of pristine MCNP prior to (black line) and after incubation in FCS at 70 °C for different times: 1 min (red line), 5 min (green line), 10 min (blue line), and 20 min (cyan line). Additionally, the key data used for the calculation of the protein content are highlighted: water content (150 °C, dashed blue line), overall weight loss (850 °C, dashed red line), and MCNP carbonate content (dashed black lines; black arrow), and (D) TEM micrograph of pristine MCNP after 5 min incubation at 70 °C.
Download figure:
Standard image High-resolution imageAll samples show zeta potentials between −20 and −30 mV after incubation compared to +47 ± 9 mV beforehand, which is a typical indication for a successful protein corona formation. There is no clear trend observable for the particle size according to temperature or incubation times, but most of the samples show a certain agglomeration tendency as the hydrodynamic diameter is increasing, which we also attribute to the repeated magnetic washing steps during which the particles are forced to agglomerate. All corresponding data can be found in table S3 in the supporting information.
The different pristine MCNP samples were applied to SDS-PAGE and silver protein staining to get a first impression on the size distribution of the corona proteins. The detected proteins were classified into three categories (low (<25 kDa), medium (25–100 kDa) and high (>100 kDa)) to get a first rough insight into the constitution and the variability of the protein corona dependent on the incubation conditions. A more detailed investigation for Apo-AI, representing the low class (<25 kDa) and albumin, representing the medium class (25–100 kDa) will be presented in section 'Effects of Nanoparticle Coating on Protein Adsorption'.
Figure 2(A) shows a pseudo false color image of the SDS-PAGE gel for different MCNP samples after incubation. The semi-quantitative analysis of protein band intensities reveals an influence of both time and temperature during incubation. With progressing duration at temperatures of both 50 °C and 70 °C an increase in the overall amount of adsorbed protein is clearly visible (figure 2(B)). The size-specific distribution of the indicated protein bands reveals that at 50 °C the ratio of low molecular weight proteins (i.e. proteins <25 kDa) increases with time from 44.1% to 51.7% predominantly in expense of decreasing medium molecular weight proteins (i.e. proteins 25–100 kDa) from 50.2% to 44.8% (figure 2(C)).
Figure 2. Determination of protein content and protein composition of pristine MCNP after incubation in FCS at different temperatures and times: (A) pseudo false color image of protein bands upon SDS-PAGE and silver staining, (B)–(C) relative optical densities of protein bands abstracted from (A), and (D) respective overall protein contents calculated from TGA measurements. The corresponding DLS and zeta potential data are also summarized in table S3.
Download figure:
Standard image High-resolution imageVice versa, at 70 °C the relative amount of high molecular weight proteins (i.e. proteins >100 kDa) increases while the fraction of proteins with molecular weights below 25 kDa is slightly reduced. Remarkably, the incubation of MCNP in FCS at 37 °C in general results in a protein corona predominantly defined by proteins below 100 kDa, as compared to all other samples here the relative amounts of high molecular weight proteins exhibit the lowest values (2.8%–3.2%).
To calculate the protein content from the TGA results, the corresponding water content at 150 °C as well as the 4% weight loss dedicated to the MCNP itself were subtracted from the overall weight losses. Prior to the incubation in FCS, MCNP showed an overall weight loss of 6.0%, of which 2.5% can be assigned to water (30 °C–150 °C). The remaining 4.0% correspond to carbonates remaining in the nanoparticle core from the synthesis. The protein contents after incubation show similar trends as obtained from SDS-PAGE. Incubation at 25 °C and 37 °C leads to protein contents between 6% and 7.5% without any clear trend for prolonged incubation times. Compared to that, the protein content after incubation at 50 °C increases with increasing incubation time from 6% to 8% and from 9% to 13.5% in case of 70 °C incubation temperature (figure 2(D)). We attribute this increase of the overall protein amount at least partially to temperature-induced denaturation, resulting in protein agglomeration and, thus, increased adsorption to the MNP surface.
PtBAA@MCNP
PtBAA@MCNP exhibited a negative zeta potential of −30 ± 6 mV and subsequent incubation in FCS leads to comparable values between −20 and −30 mV without a clear trend for incubation temperature or duration, similar as observed for the pristine MCNP. Same accounts for an apparent increase in hydrodynamic radii as observed in DLS measurements (table S4).
PtBAA@MCNP samples after different incubation times were analyzed semi-quantitatively using SDS-PAGE and silver protein staining (figure 3). At all temperatures, the overall amount of proteins increases with increasing incubation time similar to observations made for pristine MCNP at 50 °C as well as at 70 °C. If analogous incubation times at different temperatures are compared, higher protein contents are again observed for elevated temperatures. Interestingly, size distribution-specific analysis reveals that for temperatures of up to 50 °C predominantly low molecular weight proteins are adsorbed (27.5%–40.7%), whereas the ratio of medium and high molecular weight (i.e. >100 kDa) proteins simultaneously decreases from 66.2% and 6.4% to 55.4% and 3.9%, respectively. At 70 °C reversed effects are observed, as here the fraction of low molecular weight proteins decreases with progressing incubation time from 34.5% to 31.6%, while the fractions of both medium and high molecular weight proteins increase. Altogether, it can be said that similar trends concerning size distribution-specific effects are observed for PtBAA@MCNP and the pristine MCNP.
Figure 3. Determination of protein content and protein composition of PtBAA@MCNP incubated at different temperatures and times: (A) pseudo color image of protein bands upon SDS-PAGE and silver staining, (B)–(C) relative optical densities of protein bands abstracted from (A), and (D) protein contents calculated from TGA measurements where the black bars correspond to the pristine MCNP and the blue bars refer to PtBAA@MCNP. The corresponding DLS and zeta potential data are also summarized in table S4.
Download figure:
Standard image High-resolution imageWhile the overall protein content as determined via TGA does not show increasing amounts for incubation at 25 °C and 37 °C, this is different if elevated temperatures (50 °C and 70 °C) are compared, supporting the previously discussed trends obtained from silver protein staining upon SDS-PAGE. Whereas for 25 °C and 37 °C a protein content between 6.5% and 7.5% without any observable trend concerning the incubation time was found (figure 3(D)), this value increases from 7% to 9% at 50 °C and from 9% to 15% at 70 °C. Nevertheless, both the overall amount as well as the general trends are comparable to the pristine MCNP.
PDha@MCNP
After incubation, PDha@MCNP show zeta potentials between −20 and −30 mV with no clear trend for the particle size observable depending on the incubation temperature and time. As described for PtBAA@MCNP, most samples show no agglomeration, but an increased hydrodynamic radius according to DLS (table S5).
Protein adsorption on the surface of PDha@MCNP nanoparticles is verified by SDS-PAGE and subsequent silver protein staining (figure 4). The obtained data suggests increasing amounts of protein adsorbing at 25 °C, 37 °C, and 70 °C for prolonged incubation periods, and this effect is most pronounced at 70 °C. At an incubation temperature of 50 °C, however, lower amounts of protein are found. According to size-specific protein distributions, at 50 °C and 70 °C a time-dependent increase of low molecular weight proteins from 14.0% and 16.2% to 26.2% and 23.3%, respectively, can be observed while decreasing fractions of medium molecular weight proteins are present in the corona.
Figure 4. Determination of protein content and protein corona composition of PDha@MCNP incubated at different temperatures and times: (A) pseudo false color image of protein bands upon SDS-PAGE and silver staining, (B), (C) relative optical densities of protein bands abstracted from (A), and (D) protein contents calculated from TGA measurements where the black bars correspond to the pristine MCNP and the purple bars refer to PDha@MCNP. The corresponding DLS and zeta potential data are also summarized in table S5.
Download figure:
Standard image High-resolution imageMost importantly, TGA analysis shows distinctly decreased overall amounts of protein adsorbed in case of PDha@MCNP if compared to pristine MCNP and PtBAA@MCNP at all incubation conditions employed in this study. All contents vary between 4.0% and 4.5% for incubation temperatures between 25 °C and 50 °C. Only at 70 °C a certain trend from 3.5% to 5.5% adsorbed proteins with increasing incubation times can be seen (figure 4(D)).
In comparison, Saikia et al investigated protein adsorption onto silica nanoparticles of comparable size, but with negative surface charge. Here, an overall protein content of 12% was reported, which is within the same range as we observed for pristine MCNP or PtBAA@MCNP at elevated temperatures [65].
Comparison of SPION and MCNP nanoparticles
In general, during incubation in FCS at varying temperatures and for different time intervals SPION show comparable results and trends as observed for MCNP, more specific increasing amounts of protein adsorbed with increasing time and temperature for pristine nanoparticles and PtBAA@SPION, whereas reduced protein contents are found for PDha@SPION (figure 5). Compared to the results discussed for MCNP, the larger surface-to-volume ratio in case of SPIONs leads to increasing amounts of proteins adsorbed independent from the coating material. With regard to cellular cytotoxicity no effects for both SPION and MCNP coated with PDha or PtBAA were detectable by PrestoBlue™ assays using HBMEC as cell culture model as shown in the supporting information (figure S6). Interestingly, while a slight reduction in cell viability was only observed for pristine MCNP in the absence of a protein corona, pristine SPIONs still maintained this mild adverse effect also upon FCS incubation. A reason for that might be a faster dissociation and/or degradation of the protein corona on SPION surfaces but this needs to be confirmed in further studies.
Figure 5. Protein contents calculated from TGA measurements of (A) pristine SPION (black bars) and pristine MCNP (gray bars), (B) PtBAA@SPION (dark blue bars) and PtBAA@MCNP (light blue bars), and (C) PDha@SPION (purple bars) and PDha@MCNP (light purple bars). The corresponding DLS and zeta potential data are also summarized in tables S6–S8.
Download figure:
Standard image High-resolution imageEffects of nanoparticle coating on protein adsorption
All results presented above have been collected using different batches of either MCNP or SPIONs, including slight variations in either particle size or polymeric shell thickness. For further verification of the observed effects, an additional batch of MCNP samples (pristine MCNP, PtBAA@MCNP, and PDha@MCNP) was prepared and compared for selected incubation temperatures and durations (figure 6). The highest amount of protein adsorption occurs onto pristine MCNP, followed by PtBAA@MCNP, and significantly reduced protein contents are found in case of PDha@MCNP. In addition, differences detected in protein size distributions again also depend on the nature and charge of the polymeric shell. In case of pristine MCNP, medium molecular weight proteins contribute to 51%–54% of the corona, whereas in case of PDha@MCNP about 66.7%–88.5% are found with increasing tendency for elevated temperatures and incubation times. Here, the smallest fractions of low molecular weight proteins are observed as well. Regarding PtBAA@MCNP, medium molecular weight proteins are again most abundant (71.2%–74.5%), but the content of high molecular weight proteins is lower when compared to the other samples.
Figure 6. Comparison of protein content and protein composition of pristine MCNP, PDha@MCNP and PtBAA@MCNP incubated at selected temperatures and for different incubation times: (A) pseudo color image of protein bands upon SDS-PAGE and silver staining, (B–C) relative optical densities of protein bands abstracted from (A), and (D) protein contents calculated from TGA measurements.
Download figure:
Standard image High-resolution imageThese results are confirmed by TGA where the amount of proteins adsorbed to the pristine particles is distinctly higher (7.0%–12.0%) if compared to PtBAA@MCNP (5.5%–9.0%) and PDha@MCNP (0.5%–2.5%, figure 6(D)). The higher amount of proteins absorbed to the pristine particles is caused by the positive surface charge of the pristine particles compared to the negatively charged coated particles. This positive surface charge leads to a stronger electrostatic attraction of the negatively charged proteins, resulting in a higher amount of absorbed molecules, e.g. proteins.
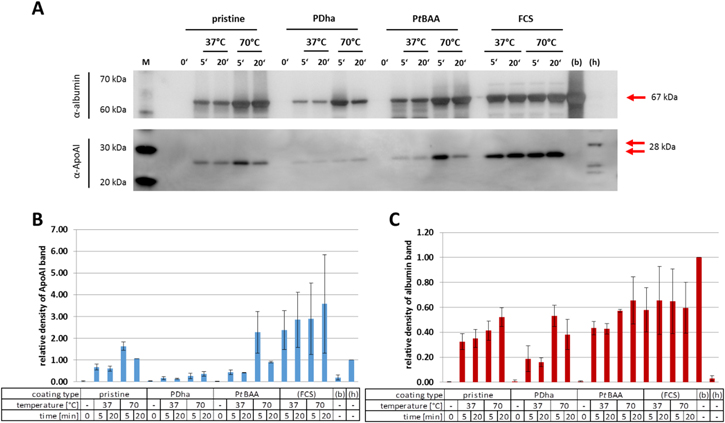
In order to get deeper insight into the protein corona composition in dependence of particle surface charge the presence of two representative proteins was quantified via Western blot assays. In particular, apolipoprotein (Apo-)AI (28 kDa) as representative of the low molecular weight fraction and albumin (67 kDa) as component of the medium molecular weight fraction were analyzed (figure 7). In agreement with the data discussed above, the amount of Apo-AI is minimal for PDha@MCNP (13.1%–36.4%), whereas increasing amounts are found of up to 227.4% (PtBAA@MCNP) and 163.1% (pristine MCNP) as normalized to HepG2 cell lysates as reference samples. Whereas the highest amounts of Apo-AI can be detected upon incubation at 70 °C (163.1% and 227.4%) in comparison to 37 °C (67.5% and 43.4%) after 5 min of incubation in case of pristine MCNP and PtBAA@MCNP respectively, prolonged incubation leads to a decrease in Apo-Al resulting in 106.8% and 90.5% for pristine MCNP and 60.6% and 41.9% for PtBAA@MCNP. Nevertheless, reference samples of FCS analogously treated with the indicated temperatures and durations imply that elevated incubation temperature and time seems to enhance the amount of Apo-AI detected via this method. Plausible explanations for this discrepancy may be the position of the antigenic determinant for the antibodies, a temperature-dependent complex formation of Apo-AI polypeptides or the elevated variability of means obtained from repetitive experiments. With regard to albumin, this effect is not observed for the FCS reference samples, where similar protein amounts are detected independent of temperature and incubation time. Analyzing albumin amounts adsorbed onto the different MCNP samples, only in case of PDha@MCNP minor contents of 18.9% and 16.9% are found for incubation at 37 °C. After incubation at 70 °C, all samples show elevated albumin contents up to 53.1% with increasing incubation time. Comparing these findings for specific proteins by Western blot analysis to the data obtained from silver protein staining, a clear correlation for albumin and Apo-Al can be observed for PtBAA@MCNP and PDha@MCNP. In case of pristine MCNP, albumin appears underrepresented and this indicates that medium molecular weight proteins other than albumin may play a critical role in the temperature- and time-dependent formation of the protein corona on the nanoparticle surface. Similarly, data found for low molecular weight proteins upon silver staining are not completely reflected by the specific detection of Apo-AI, especially in case of incubations at 70 °C for 5 min. Here, a strong over-representation of Apo-AI in the protein corona of pristine MCNP (163.1%) and PtBAA@MCNP (227.4%) compared to the silver staining-based evaluation implicates a pivotal role of Apo-AI in low molecular weight protein fractions during short-term incubations at elevated temperatures—at least for our set of samples.
Figure 7. Specific analysis of albumin and apolipoprotein AI (Apo-AI) present in protein corona of pristine MCNP, PDha@MCNP and PtBAA@MCNP incubated at selected temperatures and times: (A) immunoblots of albumin and Apo-AI including respective positive controls BSA (b) and HepG2 cell lysates (h). (B) Optical densities of Apo-AI protein bands abstracted from (A) relative to reference HepG2 cell lysates (h), and (C) optical densities of albumin protein bands abstracted from (A) relative to reference BSA (b).
Download figure:
Standard image High-resolution imageConclusion
We herein present polydehydroalanine-based materials as versatile coatings for two relevant types of magnetic nanoparticles if control over net charge and charge density is desirable. Especially PDha@SPION and PDha@MCNP are highly interesting hybrid materials, as distinctly lower amounts of proteins are adsorbed during incubation in FCS under different conditions and at varying incubation times. Regarding the used incubation conditions, at all incubation times (1–20 min) and temperatures (25 °C–70 °C) the adsorbed protein amount as determined using TGA was lower in case of PDha-based samples if compared to pristine SPION/ MCNP or samples featuring a PtBAA shell. Further, our results show that at lower temperatures (25 °C and 37 °C) the amount of protein does not increase with increasing incubation time in general whereas this is the case at elevated temperatures (50 °C and 70 °C) for pristine nanoparticles or after coating with PtBAA. This observation may be explained by the behavior of the protein albumin which is the main biomolecule component of FCS. Sahin et al [66] demonstrated that BSA exhibits a complex temperature-dependent aggregation behavior. Above 50 °C albumin aggregated irreversible due to sequential monomer addition and progressive aggregate-aggregate interactions. Semi-quantitative protein analysis of the SDS-PAGE gels supported the observations from the TGA analysis and allowed additional insight into the composition of the proteins contributing to the formed protein corona. Although both coating material and temperature seem to influence the ratios between low molecular weight proteins (<25 kD), medium molecular weight proteins (25–100 kD), and high molecular weight (>100 kD) proteins, no general trends can be derived from the obtained data so far. However, specific analysis of albumin and apolipoprotein via Western blot assays reveals that for PDha@MCNP the amount of albumin is distinctly decreased if compared to MCNP and PtBAA@MCNP at lower incubation temperatures. For the used polymers in this study no temperature-dependent structural changes, which might explain the different protein adsorption at elevated temperatures, are known. In our opinion, these findings show that PDha is a highly interesting material for the coating of magnetic nanoparticles in general and in the herein reported framework significantly decreases the adsorption of proteins from FCS samples to different types of nanoparticles. This can be of major importance for the usage of magnetic nanoparticles in medical applications where such hybrid particles have to be administered to the vascular system. There, the blood serves as a protein source and a protein corona around the particles is formed immediately and determines the biological fate of the particles within the body.
Future work will focus on the identification of participating proteins in corona formation by mass spectrometry with respect to the newly introduced surface coatings in conjunction with the incubation temperature.
Experimental part
Materials
Chemicals were purchased from Sigma-Aldrich or Carbolution (Saarbrücken, Germany) in p.a. grade and used without further purification. The photoinitiator Lucirin-TPO was kindly provided by BASF. FCS was obtained from Biochrom GmbH (Berlin) (#S0115, Lot 1184C, tested for mycoplasma and viruses, tested for endotoxins). FCS was heat-inactivated at 56 °C for 1 h prior to use.
Synthesis of MCNP
The iron oxide nanoparticles used in this paper were prepared similar to the well-known wet chemical precipitation method [67] but using a 1.17-M NaHCO3 solution as alkaline media [61]. The solution was directly added under constant stirring to a FeCl2/FeCl3 solution with a Fe2+/Fe3+ ratio of 1:1.7 with a rate of 0.9 ml min−1 and a brownish precipitate occurred. After the addition of distilled water, the particles were boiled for 5 min at 100 °C. That way multicore MNP were formed by release of CO2, and the color of the suspension turned black. Afterwards, the MNP were magnetically separated using a high-performance permanent magnet and washed with distilled water twice to remove excess educts. To avoid aggregation and to stabilize the particles in suspension 200 μl of 1M HCl have been added and the suspension has been treated with ultrasonication for 1 min (Sonopuls GM200, BANDELIN electronic, Berlin, Germany).
PtBAMA
A solution of 58 mg (0.168 mmol) TPO in 6 ml 1,4-dioxane was added to 6 g (19.8 mmol) of tBAMA (M:I = 200:1). The mixture was placed in an UV-cube (250 W) for 5 min. The polymer was precipitated in a mixture of ethyl acetate and hexane (1:4). Yield: 52%
1H-NMR: (300 MHz, CDCl3): δ = 5.4 (b, 1H, NH), 3.7 (3H, OCH3), 1.4 (9H, Boc).
PtBAA
500 mg of PtBAMA were dissolved in 10 ml 1, 4-dioxane and a solution of 2.5 g LiOH (∼45 equation) in 10 ml H2O was added. The mixture was stirred at 80 °C for 3 h freeze dried and dialyzed against water. Yield: 90%.
1H-NMR: (300 MHz, D2O/NaOD): δ = 5.7 (b, 2H. CH2), 1.3 (s, 7H, Boc),
PDha
500 mg of PtBAMA were dissolved in a mixture of 1 ml H2O and 4 ml trifluoroacetic acid (TFA) and stirred for 1 h at 50 °C. The product was precipitated in methanol. The resulting solid was dissolved in 10 ml 1, 4-dioxane and a solution of 2.5 g LiOH (∼45 equation) in 10 ml H2O was added. The mixture was stirred at 100 °C for 3 h and neutralized with diluted HClaq. During the neutralization, PDha precipitated. Yield: 97%.
PtBAA@MNP
3.2 g PtBAA were dissolved in 160 ml water/NaOH mixture at pH = 12. The solution was carefully brought to pH = 5 with 0.1 M HCL. 800 mg of the respective MNP dispersion (20 g l−1, 40 ml) were added to the solution and the mixture was ultrasonicated for 1 h. The particles were magnetically separated and washed with micropure water five times.
PDha@MNP
3.2 g PDha were dissolved in 160 ml water/NaOH mixture at pH = 12. The solution was carefully brought to pH = 5 with 0.1 M HCL. 800 mg of the respective MNP dispersion (20 g l−1, 40 ml) were added to the solution and the mixture was ultrasonicated for 1 h. The particles were magnetically separated and washed with micropure water five times.
Incubation
1 ml of FCS was given separately in each of five 2 ml vessels placed in a tempered water bath at the respective incubation temperatures of 25 °C, 37 °C, 50 °C and 70 °C to guarantee a homogeneous temperature distribution throughout the samples. After applying 750 μl of the previously prepared MCNP suspension (concentration 10 g (MCNP)/L, 7.5 mg of MCNP) to every sample, incubation was performed for one of the respective times (1, 5, 10 or 20 min). During this time the suspensions were treated with ultrasound in an accordingly tempered ultrasonic bath (US-bath, S100H, Elmasonic, Germany) to re-disperse possible agglomerates at several time points (for details see table S2). Afterwards, the samples were taken out of the water bath and were magnetically separated. The excess FCS was withdrawn and 1 ml of distilled water was added. Afterwards the particles were treated again with ultrasound for 1 min and this washing procedure was repeated for further four times to remove any loosely bound proteins. Afterwards, the washed MCNPs of the resulting samples have been mixed together in a glass vessel, ultrasonicated for 1 min and the final samples for characterization were taken (approx. 0.6 ml for zeta measurement, DLS and VSM; approx. 1.6 ml for SDS-PAGE and cytotoxicity studies and 2 ml for TGA and TEM-measurements). After incubation, the samples were stored in the fridge at 4 °C.
Protein analysis via silver staining
The overall analysis of proteins present within the protein corona was performed by SDS-PAGE followed by silver staining as described before [68, 69]. In brief, 8.0 μg nanoparticle formulations prepared as described in the previous section were diluted in lysis buffer (20 mM HEPES, 150 mM NaCl, 10 mM EDTA, 2 mM EGTA, 1% (v/v) Triton X-100, 10 mM Na4P2O7, 50 mM NaF, 2 mM Na3VO4, 10 μg ml−1 aprotinin, 1 μM pepstatin, 10 μM leupeptin, 500 μg ml−1 pefabloc®) and mixed with 4 × XT sample buffer and 20 × Reducing Agent (both Bio-Rad, Hercules, USA). Heat denaturation was carried out for 5 min by incubation at 95 °C before samples were loaded on a 4%–12% Bis-Tris (tris(hydroxymethyl)aminomethane) gel (Bio-Rad). Upon electrophoretic protein separation (45 V, maximal 300 mA, 90 min) according to molecular sizes along with a Kaleidoscope protein standard ladder (Bio-Rad), protein bands were visualized by silver staining (SilverXpress Silver Staining Kit, Thermo Fisher Scientific, Waltham, USA). Acquired gray-scale gel images were converted to alternative color maps by MATLAB® (MathWorks, Natick, USA). Semi-quantitative analysis of optical protein band intensities were performed using ImageJ 1.50e basic version (Wayne Rasband, National Institutes of Health, USA).
Protein analysis via Western blot assay
For the specific detection of proteins SDS-PAGE was performed as stated above. After gel run, proteins were blotted on PVDF Trans-Blot® membranes (Bio-Rad) using the Trans Blot® Turbo Transfer system (Bio-Rad) with a constant current of 1.0 A for 30 min at a maximum of 25 V. Protein studded PVDF membranes were blocked with 5% (w/v) skimmed milk blocking buffer (20 mM TBS (Tris-buffered saline), 0.1% (v/v) Tween® 20) and incubated with Apo-AI- (#A55259, EpiGenTek, Farmingdale, USA) or albumin- (#A11133, Thermo Fisher Scientific) binding antibodies diluted 1:1000 in 5% skimmed milk blocking buffer at 4 °C over night. After three washing steps using TBS-T (20 mM TBS, 0.1% (v/v) Tween® 20) membranes were incubated with secondary horseradish-labeled α-rabbit IgG antibodies (#7074, Cell Signaling Technologies, Danvers, USA, 1:1000 diluted in TBS-T). Upon another three washing steps, chemiluminescent signals were detected at the LAS4000 (GE Healthcare Life Science, Buckinghamshire, UK) via ECL reagents (Thermo Fisher Scientific). Semi-quantitative analysis of optical protein band intensities was performed using ImageJ 1.50e basic version.
PrestoBlue™ cell viability assay
Cell viability studies upon exposure to the specified particle formulations were performed utilizing HBMEC. Cells seeded into black-walled 96-well plates (μ-Clear®, F-bottom, Greiner Bio-One, Frickenhausen, Germany) in triplicate were incubated with particle concentrations between 5–100 μg cm−2 (corresponding to 19–378 μg ml−1), water (negative control), or polyethylenimine-coated PEI-M particles (positive control, micromod Partikeltechnologie GmbH, Rostock, Germany). After 3 and 24 h PrestoBlue™ cell viability Reagent (Thermo Fisher Scientific) was added and incubated at 37 °C for further 30 min. After annular magnetic sedimentation of particles to the well periphery fluorescence at 600 nm (40 nm bandwidth) was detected upon excitation with 545 nm (20 nm bandwidth) using the CLARIOstar microplate reader (BMG LABTECH GmbH, Orthenberg, Germany). Nanoparticle-associated auto-fluorescence effects were verified by carrying along cell-free wells treated with particles. Furthermore, quenching effects were monitored by comparison of fluorescent signals before and subsequently after particle addition into cell-seeded control wells.
Instrumentation
Size exclusion chromatography in CHCl3: SEC measurements were performed on a Shimadzu system equipped with a SEC SCL-10A system controller, a LC-10AD pump, and a RID-10A refractive index detector using a solvent mixture containing chloroform, triethylamine, and isopropanol (94:4:2) at a flow rate of 1 ml min−1 on a PSS-SDV-linear M 5 μm column at 40 °C. The system was calibrated with PMMA (410–88 000 Da) standards. Zeta potential measurements: The zeta potentials of the samples were measured on a ZetaSizer Nano ZS from Malvern via M3-PALS technique with a laser beam at 633 nm. The detection angle was 13°. Malvern Software (version 7.11). Dynamic light scattering: DLS measurements were performed using an ALV Laser CGS3 Goniometer equipped with a 633 nm HeNe Laser. DLS measurements were performed at 25 °C and at a detection angle of 90°. The CONTIN algorithm was used to evaluate the obtained data. Additionally, DLS measurements for the pure and incubated particles were performed by Zetasizer Nano ZS from Malvern using NIBS technology and Malvern Software (version 7.11). Transmission electron microscopy (TEM): For TEM from aqueous solutions, copper grids were rendered hydrophilic by Ar plasma cleaning for 30 s (Diener Electronics). 15 μl of the respective sample solution were applied to the grid and excess sample was blotted with a filter paper. TEM images were acquired with a 200 kV FEI Tecnai G2 20 equipped with a 4 k × 4 k Eagle HS CCD and a 1 k × 1 k Olympus MegaView camera for overview images. UV-Irradiation was carried out using a Hoehnle UVACUBE 100 equipped with a 250 W lamp. Ultrasonication was performed using an ElmaSonic S30H ultrasonic unit. Thermogravimetric Analysis: The samples were washed five times with distilled water as described in the incubation section. Afterwards samples were magnetically separated from the supernatant, frozen and subsequently freeze dried for 72 h. TGA measurements were carried out from 30 °C up to 800 °C under synthetic air with a heating range of 10 K min−1 in a Perkin Elmer TGA8000 device.
To calculate the protein content of the incubated nanoparticles, the TGA data of three particle samples are needed: The corresponding pristine particles, the corresponding polyelectrolyte coated particles, and the incubated particles itself. First, the pristine samples were measured to determine the carbonate content of the particles. Therefore, the weight loss at 150 °C (residual water) was deducted from the overall weight loss of the pristine particles. Subsequently, the polyelectrolyte coated particles were investigated to determine the polyelectrolyte content. In this case, both the water content of each sample (weight loss at 150 °C), and the previously determined carbonate content of the corresponding pristine particles was deducted from the overall weight loss. Finally, to determine the protein contents, each incubated particle sample was measured, and the weight loss at 150 °C, the corresponding polyelectrolyte content and the corresponding carbonate content were deducted from the overall weight loss.
From the determined protein amount, the protein shell thickness was calculated following equation (1):
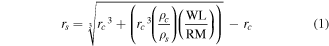
with
- rs shell thickness
- rc core radius
- ρc core density
- ρs shell density
- WL weight loss dedicated to shell
- RM residual mass
Acknowledgments
F Schacher, S Dutz, A Weidner, C Gräfe, J Clement and M von der Lühe are grateful to the Deutsche Forschungsgemeinschaft for financial support in the framework of the SPP1681 (DFG, FKZ: DU 1293/7-3, CL202/3-2, SCHA1640/7-1). The cryo-TEM/TEM facilities of the Jena Center for Soft Matter (JCSM) were established with a grant from the German Research Council (DFG) and the European Funds for Regional Development (EFRE).