Abstract
The design and controlled synthesis of two-dimensional (2D) nanomaterials have been widely studied because the properties and functions of nanomaterials are highly dependent on their sizes, shapes, and dimensionalities. For instance, 2D metal nanosheets (2DMNSs) have attracted a significant amount of attention owing to their interesting properties, which are absent in corresponding bulk counterparts, and they have been confirmed to have potential applications in electrocatalysis, optics, and biomedicine. However, because of the close-packed structures of metals, the large-scale fabrication of 2DMNSs is challenging. In this review, we have outlined the research progress in the field of 2DMNSs, including the typical synthesis approaches and newly developed methods, as well as promising applications of the materials reported in recent years. Moreover, some preliminary and promising strategies to further improve the properties of 2DMNSs and some insights for the development of the field have been included.
Export citation and abstract BibTeX RIS
1. Introduction
Two-dimensional (2D) materials have been extensively studied in the field of nanomaterials owing to their distinct optical, electronic, and chemical properties arising from their ultrathin nature. Since the first report on graphene in 2004, several 2D materials have been exploited including metal dichalcogenides [1–3], metal nitrides [4, 5], metal phosphides [1, 6, 7], 2D boron nitrides [8], 2D MXenes [9, 10], 2D metal organic frameworks [11–14], and pure metals, which are mentioned in this paper. 2D nanosheets (NSs) exhibit unique properties, which are essentially absent in a corresponding bulk counterpart; these properties are attributed to the absence of the third dimension in the 2D materials. For instance, because of the ultrathin nature of NSs, and their high surface area to volume ratios, the number of active sites is close to the total number of atoms, which render them with efficient catalytic properties [15, 16]. Notably, 2D transition metal dichalcogenides possess promising optical and electrical properties because of the suitable bandgaps (around 1.0–2.0 eV) [17, 18]. Moreover, it has been proven that the lengths and thicknesses of NSs have an obvious effect on their electrochemical performances [19]. The ultrathin structure endows the NSs with great potential for extensive application in various fields, including energy storage [9], field-effect transistors [20], and in particular, catalysis [21–23]. Reviews on most 2D materials including 2D transition metal phosphides [24, 25], 2D metal chalcogenides [26], 2D transition metal dichalcogenides [27, 28], 2D metal organic frameworks [29, 30] and 2D Au sheets [31] have been extensively published.
Some materials naturally possess layered structures, wherein the layers are held together by weak interlayer van der Waals forces. 2D NSs of such materials can be directly extracted from their parent materials by mechanical and chemical exfoliation [32–34]. However, metallic atoms display a natural tendency to form 3D close-packed structures because of the non-directional nature of the metallic bonds. As a result, preparation of 2D metal nanostructures via direct delamination is infeasible in most cases. The difficult preparation of 2D metal NSs (2DMNSs) has seriously hindered their property studies; on the other hand, because of the ease of synthesis, various metal nanoparticle catalysts have been extensively prepared, and the influence of composition and microstructure of metal nanoparticles on their catalytic properties has been vastly studied [35–40].
Initially, methods based on layer-by-layer deposition of metal atoms were applied to synthesize various metal films with tens or hundreds of nanometer thicknesses, such as physical vapor deposition [41], molecular beam epitaxy [42–44], chemical vapor deposition [45, 46], magnetron sputtering [47, 48] and atomic layer deposition [49]. These films could be regarded as initial 2D metal materials. Although the purity and thicknesses of the metal films could be controlled to a certain degree [50–52], the films contained impurities, had low crystallinities, and were tightly attached to the substrates [53]. Moreover, reports on 2D metal films with thicknesses less than 10 nm were rare, even if the thicknesses could be controlled by number of deposition cycles via atomic layer deposition [52]. Furthermore, some metal films with high thicknesses could not be considered 2DMNSs because they consisted of nanoparticles and grain boundaries [49].
However, in the last ten years, various methods including solution-based chemical approaches, electronic exfoliation, and mechanical method have been successfully developed to fabricate 2D metal materials with a few nanometer thicknesses from metal salts [23, 54–65], bulk metals [66], metal particles [67], and metal foils [68], (scheme
Scheme 1. Approaches to obtain 2D metal nanosheets. [66, 67] John Wiley & Sons, © 2017 Wiley‐VCH Verlag GmbH & Co. KGaA, Weinheim. [68] John Wiley & Sons, © 2016 Wiley‐VCH Verlag GmbH & Co. KGaA, Weinheim.
Download figure:
Standard image High-resolution image2. Approaches to obtain 2DMNSs
2.1. Solution-based chemical approaches
Usually, in solution-based chemical synthesis, reagents, such as solvents and reactants, and the reaction conditions, such as pressure, temperature, and pH, are easy to be controlled. In addition, the raw materials are well dispersed in a solvent to facilitate the reactions. As a result, solution-based chemical approaches including the wet chemistry method, hydrothermal and solvothermal methods, and template method have been widely used to prepare various kinds of 2D materials, including 2DMNSs [2–7].
In these methods, generally, metal precursors and organic agents are used as the starting materials. To obtain stable 2DMNSs, two common strategies have been developed. The first one involves the use of organic directing agents to control the growth orientation during the precursor reduction. The commonly used organic directing agents include a series of organic complexing agents such as 1-amino-9-octadecene [55], formate [23], acetonitrile [56], trisodium citrate, ethylenediaminetetraacetic acid disodium salt [54], and polyvinyl pyrrolidone [56]. Moreover, to reduce the metal precursors, the following reducing agents have been used: formate [23], hydrazine [54], ascorbic acid, and H2 [55]. Generally, when a precursor compound is uniformly dispersed in a solvent, first, the metal ions are reduced by the reducing agent, forming a metal nucleus. Organic molecules serve as structure-directing agents and control the addition of adsorptive atoms to the specific crystal faces by strongly adsorbing on certain surfaces of the metal nucleus; consequently, the primary metal particles directionally assemble, finally, leading to the formation of stable 2D structures [71]. Moreover, because organic molecules can serve as capping agents or growth-directing agents for NSs, by using them, the size and the morphologies of the final products can be well controlled [54, 57, 72]. The second strategy to obtain 2DMNSs involves the application of 2D templates and paving metal atoms onto surface of templates for the growth of the materials. In this method, reduced metal atoms spread and grow on the surface of templates such as graphene oxide (GO), Ni(OH)2 NSs, other metal NSs [23, 55, 56], etc, leading to the formation of 2DMNSs on the templates. In short, most 2DMNSs have been well prepared by solution-based chemical approaches, which are the most widely followed routes.
2.1.1. Conventional wet chemistry method
As one of the most classic methods, the conventional wet chemistry method under general conditions has been extensively used for the synthesis of MNSs with 2D morphology and structure. Typically, to obtain well dispersed 2DMNSs in the reducing progress, organic surfactants such as oleic acid [59] and octadecene [55] are introduced into the reaction system as capping agents and structure-directing agents, which can restrict and control the growth of metal NSs.
In 2008, Cao's group [54] prepared silver NSs at room temperature by reducing silver oxide with hydrazine in the presence of trisodium citrate and ethylenediaminetetraacetic acid disodium salt dihydrate (EDTA) in aqueous conditions. Particularly, the shapes and edge lengths of the Ag NSs were well controlled by altering the ratio of the EDTA salt to Ag+ (table 1). The resulting Ag NSs clearly demonstrated surface plasmon resonance (SPR), which was in a linear relationship with the quantity of EDTA, indicating that EDTA played a role in controlling the microstructure of the Ag NSs. Thus, they successfully demonstrated that the property of NSs could be tuned by precisely controlling the product morphology and size. In 2016, Bu and coworkers [70] synthesized 2D PtPb/Pt core/shell NSs using platinum acetylacetonate (Pt(acac)2) and lead acetylacetonate (Pb(acac)2) as the metal precursors, an oleylamine/octadecene mixture as the surfactant and solvent, and ascorbic acid as the reducing agent. The dominant product was hexagonal nanoplates with a thickness of 4.5 nm; the thickness of Pt shell layer formed at the edge was about 0.8–1.2 nm. The effect of synthetic parameters including precursor, surfactant, and reducing agent on the formation of structurally ordered intermetallic PtPb/Pt NSs were investigated in detail. However, the growth mechanism, including the reduction of metal species, initial formation of metal nucleus, and interaction of structure-directing agents and metal were not clearly understood. Notably, metal-ligand interactions often affect the reduction rates and growth modes, and subsequently, lead to the formation of MNSs with different shapes; therefore, a clear understanding of growth mechanism is essential for the design and preparation of desired 2D NSs.
Table 1. Average edge lengths, thickness, and SPR band maxima of Ag nanoplates obtained with different amounts of EDTA. Reproduced from [54] with permission of The Royal Society of Chemistry.
| EDTA/μl | 30 | 50 | 70 | 90 |
| SPR/nm | 596 | 700 | 851 | 852 |
| Shape | Triangle | Triangle | Hexagon | Hexagon |
| Edge length/nm | 40 ± 8 | 85 ± 8 | 100 ± 20 | 145 ± 20 |
| Thickness/nm | 11.2 ± 1.5 | 9.0 ± 0.8 | 8.5 ± 0.4 | 13.6 ± 1.2 |
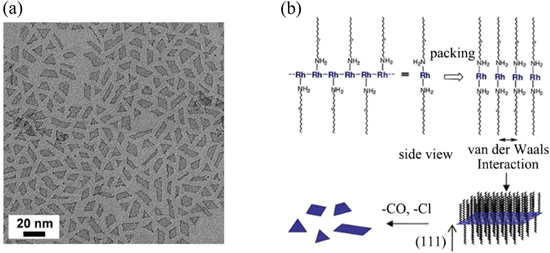
Notably, some remarkable studies focused on revealing the various mechanisms in specific syntheses of 2DMNSs, especially the role of capping agent, have made substantial progress. In 2010, Jang's group [57] firstly prepared ultrathin (1.3 nm in thickness) rhodium NSs (figure 1(a)) by heating the [Rh(CO)2Cl]2 and oleylamine solution at 50 °C for 10 days without stirring. On the basis of the UV–vis spectroscopy analysis, they attributed the formation of the NSs to the chain structure of Rh+ via metal-metal interaction between Rh+ precursors. According to the proposed mechanism, the coordinated oleylamines located above and below the chains, and the chains interact with each other via van der Waals forces between alkyl groups to form a lamellar structure. Subsequently, the gradual reduction of Rh+ to Rh results in the formation of 2D NSs (figure 1(b)).
Figure 1. (a) TEM image of rhodium nanosheets. (b) Schematic of the formation mechanism of the nanosheets. Reprinted with permission from [57]. Copyright (2010) American Chemical Society. Reprinted with permission from [57]. Copyright (2014) American Chemical Society.
Download figure:
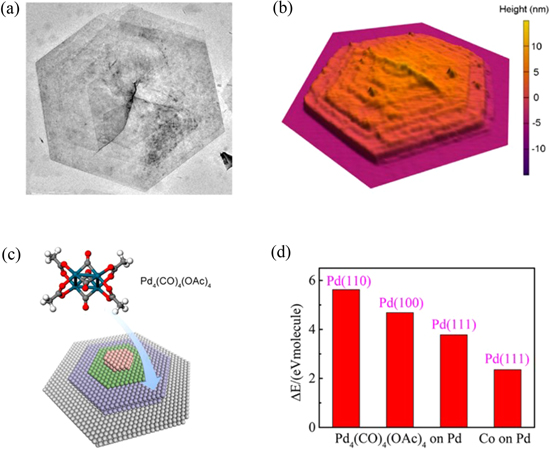
Standard image High-resolution imageIn 2010, Yang's group [71] synthesized Hanoi Tower-like Pd NSs with thicknesses approximately 10 nm by dissolving Pd(acac)2 in acetic acid (HOAc) and maintaining the solution under bubbling carbon monoxide (CO) gas at room temperature for 24 h. To explore the mechanism, density functional theory (DFT) calculation with van der Waals correction was performed to calculate the adsorption of the Pd4(CO)4(OAc)4 complex and CO on Pd(110), Pd(100), and Pd(111) surfaces. Because the Pd4(CO)4(OAc)4 complex occupied at least two atomic sites on the Pd surface and CO was adsorbed only on one atomic site, thus the adsorption strength of the complex on the surface was twice that of CO. As a result, the adsorption of Pd4(CO)4(OAc)4 complex on Pd(111) was inhibited by CO and on Pd(100) was just slightly favored over CO while on Pd(110) was much favored. Thus, the preferred adsorption of the Pd4(CO)4(OAc)4 complex on the Pd(110) surface led to the anisotropic growth of Pd NSs along the 〈110〉 directions and inhibition along 〈111〉 directions (figure 2). Later, the group discovered a unique formation mechanism of Pd NSs with dish-like morphologies [72]. The product was prepared by immersing a mixture of oleylamine, oleic acid, and Pd(acac)2 in an oil bath at 130 °C under magnetic stirring and CO bubbling. They proposed that the oriented 2D growth and the formation of the dish-like structures were related to the (211) twinning on the sides of the (111) planes of face-centered Pd, which restricted further growth of the basal plane. Subsequently, the epitaxial growth on the (111) plane was favored, leading to the formation of the sides of the nanodish, and finally, the dish-like morphology.
Figure 2. (a) TEM image of multilayered Pd nanosheets. (b) AFM 3D topography. (c) Illustration of the formation of multilayered Pd nanosheets from Pd4(CO)4(OAc)4. (d) DFT calculated Ead of Pd4(CO)4(OAc)4 complex on various Pd surfaces. Reprinted with permission from [71]. Copyright (2014) American Chemical Society.
Download figure:
Standard image High-resolution imageIn addition to the conventional organic directing agents, CO was proven effective in controlling the anisotropic growth of 2DMNSs and the detailed mechanism was explored. Using palladium(II) acetylacetonate as the precursor and DMF as the solvent, Zheng and coworkers [64] obtained free-standing Pd NSs under 1 bar CO atmosphere in a glass pressure vessel by heating the solution at 100 °C for 3 h with stirring. In the synthesis, CO acted as a blocking agent, playing a critical role in controlling the formation of Pd NSs. A dominant peak at 1.024 V (versus reversible hydrogen electrode) attributed to CO stripping from Pd(111) were observed in the CO stripping voltammetric curve. This result verified the assumption that the strong adsorption of CO molecules on the basal Pd(111) planes was responsible for restriction of growth along the [111] direction. Owing to the growth confinement effect of the CO, the average thickness of the Pd NSs was restricted to 1.8 nm (less than 10 atomic layers), and the edge length was synthetically controlled from 20 to 160 nm. By the same mechanism, they also synthesized 2D rhodium NSs at 150 °C in a CO atmosphere. The thicknesses of the individual Rh NSs ranged from 7.5 to 11.0 nm and the long-edge lengths of the parallelogram sheets were between 450 nm to 880 nm [63].
To date, the conventional wet chemistry method has been extensively used in the preparation of MNSs. Although this method is easy to perform because of its moderate reaction conditions, it is ineffective for low dynamic synthesis and it usually involves long reaction times. With regard to these features, the hydrothermal and solvothermal methods are more efficient.
2.1.2. Hydrothermal and solvothermal methods
In the hydrothermal and solvothermal syntheses, which are performed under high temperature and high-pressure conditions, chemical reactions that do not occur under normal conditions can easily proceed. Accordingly, these methods have been used for the synthesis of some 2DMNSs. In typical hydrothermal and solvothermal syntheses, first, metal precursors, surfactants, and solvents are homogeneously mixed. Then, the resulting mixture is transferred to a glass pressure vessel to achieve the desired conditions and held for hours. The size, shape distribution, and crystallinity of the products can be controlled by altering certain experimental parameters, including the precursor, surfactant, solvent, pH value, reaction temperature, and reaction time. More studies involving the hydrothermal and solvothermal methods than the conventional wet chemistry method have been reported. In addition, the roles of the aforementioned synthesis parameters have been well explored, providing significant understanding of the growth mechanism of 2DMNSs
In 2012, Yin's group [60] reported the shape-selective hydrothermal synthesis of ultrathin triangular and irregular Ru NSs with narrow size distribution through reduction of RuCl3·xH2O using formaldehyde. They suggested that the intrinsic growth of Ru nanocrystals under suitable thermodynamic conditions led to the exposure of the most stable (0001) facets for surface free energy reduction, resulting in the formation of Ru NSs When the concentration of the Ru precursor was increased, thinner NSs with irregular shapes were obtained due to the relatively faster nucleation and growth, indicating that the morphology of the NSs could be well controlled on the basis of nucleation growth mechanism.
Structure-directing agents have also been reported to play crucial roles in the hydrothermal and solvothermal syntheses of some 2DMNSs. In 2014, Duan's group [65] reported the fabrication of polyvinyl pyrrolidone (PVP)-supported single-layered rhodium NSs with hexagonal structure by a facile solvothermal method at 180 °C for 8 h. Atomic force microscopy showed that the thickness of a rhodium NS was <4 Å. Electron diffraction and x-ray absorption spectroscopy analyzes revealed that the product was composed of planar single-atom-layered sheets of rhodium. Notably, DFT calculation indicated that the formation of conjugated δ-bonds between the Rh surface and PVP led to partial saturation of the dangling bonds on the Rh surface, which decreased the surface energy of the single-layered Rh sheets to a certain extent. Moreover, the conjugated d-bonding between PVP and the specific Rh surface enhancement the stability of the single-layered Rh sheets. In 2017, Mahmood and coworkers [61] also prepared trimetallic PtAgCo alloy NSs by a robust solvothermal method. The decomposition of formaldehyde for a long time at high temperature produced CO, which acted as a reducing agent for the precursors. First, twinned Pt/Ag particles and multipods were formed, from which, finally, NSs were formed. Because the edge faces of the Pt/Ag NSs had higher surface energies than the basal planes, Co preferentially deposited on the edges and acted as active sites, promoting further growth of Pt. Moreover, CO acted as a reducing agent, which strongly bound onto the (111) faces, maximizing the (111) surface exposure, and confined the growth of NSs. These two factors led to the anisotropic growth of PtAgCo NSs with Pt-enriched edges.
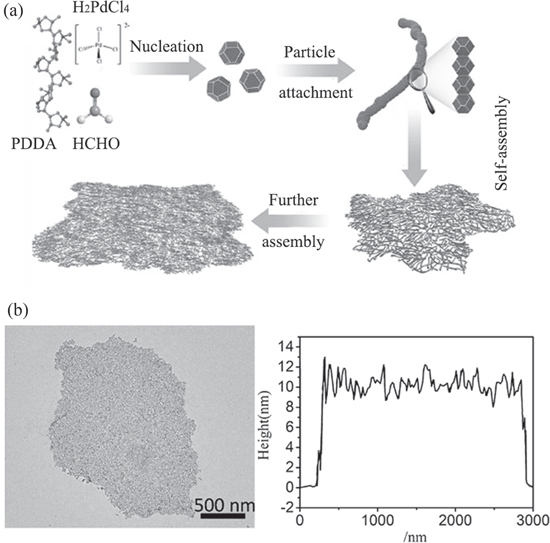
The influence of pH on the growth of NSs was first reported by Qiu's group [62]. They hydrothermally reduced the Pd precursor K2PdCl4 with HCHO in the presence of poly(dimethyldiallylammonium chloride) (PDDA) at pH 11 and successfully synthesized free-standing Pd NSs. In this one-pot method, Pd NSs were formed via the spontaneous attachment of particles, and the subsequent self-assembly of the 0D nanoparticles to form 2D NSs in the presence of the structure-directing agent PDDA (figure 3). Notably, when the pH was varied, no 2D NSs were detected, indicating that the growth of the NSs was especially sensitive to the pH value. Similarly, they synthesized PdAu and PdCu alloy NSs, confirming the versatility of the method for the preparation of Pd-based NSs Although, the influence of pH value is reported, the underlying mechanism is not clearly understood and it needs to be explored further.
Figure 3. (a) Schematic of the formation mechanism of free-standing 2D porous Pd nanosheets through particle attachment and self-assembly. (b) TEM image and the height profile of Pd nanosheets. [62] John Wiley & Sons. © 2016 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.
Download figure:
Standard image High-resolution image2.1.3. Template method
Because the formation of most NSs depends on the modulation of the capping effect of ligands, much success has been not been achieved, and particularly, tuning of the NS morphology has been challenging. To overcome this issue, a template method has been developed via the epitaxial growth of metals on specific templates. The introduction of the templates has afforded the synthesis of NSs of a broad range of metals, and enabled the control of NS thickness at the nanoscale.
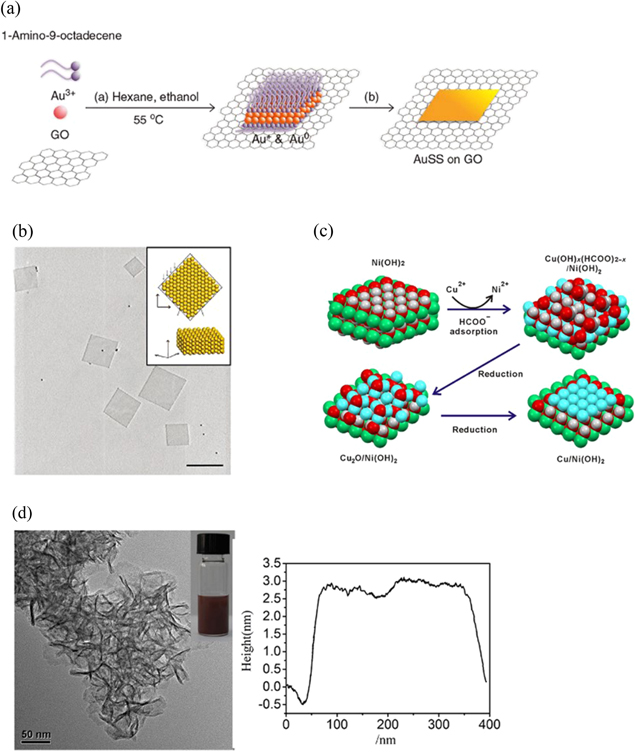
GO, a well-known 2D material, has been widely used as a template. In 2011, Huang's group [55] reported the in situ synthesis of pure hexagonal ultrathin Au sheets (figure 4(a)) on GO. When 1-amino-9-octadecene and Au3+ hexane were mixed with GO sheets, Au3+ was partially reduced to Au+, and subsequently, the 1-amino-9-octadecene-AuCl complex was formed on the GO surface. After addition of ethanol to the solution and heating at 55 °C for 8 h, the complex self-assembled into square-like structures through aurophilic interactions. Finally, the formed small Au seeds grew and fused with each other, resulting in the formation of Au NSs with a square-like morphology. The thickness of the final NSs was estimated to be 2.4 nm (figure 4(b)). Later, in 2015, using the as-prepared Au NSs (edge length of 100–400 nm and thickness of ∼1.8 nm) as templates, they further synthesized ultrathin fcc (101)-oriented Au@Pt and Au@Pd core–shell rhombic nanoplates through the epitaxial growth of Pt or Pd on the Au NSs [73]. Typically, Au@Pt NSs were prepared by successively adding, hexane, ethanol, NaBH4 solution, and H2PtCl6 solution (2 mM, ethanol) into the as-prepared Au NS solution (in hexane). Then, the mixture was gently shaken and then left undisturbed under ambient conditions for 3 h. The final product was collected by centrifugation and washed once with hexane. Au@Pd NSs were synthesized in the same manner by using the H2PdCl4 solution (8 mM, ethanol) as the metal precursor. However, the application of graphene as a template instead of Au in the synthesis of ultrathin Pt or Pd nanostructures can limit the structural diversity and reduce the total cost.
Figure 4. (a) Schematic of Au square sheet fabrication. (b) TEM image of Au 2DMNSs on a GO surface (scale bar = 500 nm). (c) Schematic of the preparation of hybrid Cu/Ni(OH)2 nanosheets. Color codes: cyan, Cu; green, Ni; red, O; gray, C; and white, H. (d) TEM image and the height profile of Cu/Ni(OH)2 nanosheets. (a) and (b) [55] © 2011, with permissions of Springer Nature. (c) and (d) from [69]. Reprinted with permission from AAAS.
Download figure:
Standard image High-resolution imageIn 2017, Liu's group [56] successfully synthesized Pt NSs by reducing the H2PtCl6/CH3CN complex on a Ag template using a combination of deprotonated ascorbic acid and H2. It made templates for Pt nanostructures much more diverse in morphology. Ag templates were synthesized by injecting NaBH4 solution (0.1 M, 1.2 ml) into a solution containing trisodium citrate dihydrate (0.075 M, 12 ml), AgNO3 (0.1 M, 200 ml), H2O2 (30%, 480 ml), and 200 ml of H2O under vigorous stirring for 30 min. Notably, Pt NSs were obtained by successfully controlling reaction kinetics and thermodynamics. In detail, coordination of the chloroplatinate salt with CH3CN lowered the rate of reduction of the Pt salt, which led to the migration of Pt atoms to distant regions to form ultrathin NSs. Via the introduction of deprotonated ascorbic acid and high temperature H2, a strong reductive environment was created, which led to the favorable reduction of the H2PtCl6/CH3CN complex, and thus, competitive kinetics of the growth of Pt NSs against the thermodynamically tendency for galvanic replacement of Ag. Moreover, PVP acted as a capping agent and enhanced the stability of the Ag Ns, further restraining the galvanic replacement. As a result, epitaxial crystal growth of Pt on the Ag template was achieved, and the surface structure of ultrathin Pt nanoplates could be controlled by using the Ag template. Finally, Pt NSs with thickness of 1–2 nm were successfully collected after the selective etching of Ag template with nitric acid through the pinholes that enabled intermixing of Pt and Ag at the incipient stage of the epitaxial growth.
In addition to the epitaxial growth method, replacement reaction between target metal ions and templates has been prove to be an effective route to synthesize 2DMNSs. In 2017, Dai's group [69] synthesized 2D Cu NSs with thicknesses less than 3 nm using Ni(OH)2 NS templates. In detail, by mixing NiCl2, Cu(acac)2, and HCOONa with N,N-dimethylformamide in a glass pressure vessel and heating at 160 °C for 10 h, Ni(OH)2 NSs were first prepared. Then, Ni2+ was replaced by Cu2+ because the solubility product constant (Ksp) of Cu(OH)2 (5.6 × 10−20) was much smaller than that of Ni(OH)2 (5.5 × 10−15). Finally, Cu(OH)2 within the NSs was reduced with formate, leading to the formation of 2D Cu NSs. Because the whole reduction process occurred within the NSs, the reduced Cu species were confined as NSs within the hybrid NS template (figures 4(c) and (d)). Similarly, in 2018, via the galvanic replacement strategy and using Ag NSs as templates, Au@AuAg yolk-shell NSs [74] and independent porous Pd, Au, Pt NSs [75] were synthesized.
Remarkable success has been achieved in controlling the sizes and morphologies of ultrathin 2DMNSs via the template method. However, there is still scope for extensive research because the method could be successfully applied to only a few types of metals. Application of this method to obtain a wider range of 2DMNSs can be considered a research topic in future studies. Moreover, this method can either be used to obtain hybrid 2DMNSs (e.g. Au@Pt) or individual 2DMNSs (e.g. by etching Ag template to synthesize Pt NSs) 2DMNSs attached to templates are not suitable for applications requiring the properties of the both the materials. Therefore, template etching is a critical process, and various methods are used, depending on the character of the template. Hence, to extend the scope of the template method, it is significant to select a proper template that can be easily separated from the target NSs.
In general, solution-based chemical approaches are the most widely used methods for the synthesis of ultrathin 2D metallic nanomaterials. Remarkable success has been achieved in controlling the sizes and morphologies of ultrathin 2D metallic NSs via these synthetic strategies using surfactants or templates. Moreover, these methods, which make use of the strong surface confinement capability of capping agents to control the growth of materials in two dimensions, are effective strategies to synthesize 2D NSs. Currently, the application of these methods is limited to the synthesis of specific kinds of metal NSs. However, fabrication of ultrathin 2D NSs of various other metals by using new blocking agents or by exploiting the unique physical and chemical nature of the metals should be possible. We believe that in the near future the studies currently being performed can lead to new developments in the field. Nonetheless, the solution-based methods have some limitations. For instance, environmentally unfriendly or harmful chemicals including organic agents, toxic metal salts, poisonous gases, etc are used in these methods. Moreover, the synthesis processes are usually complicated, and the precise control of the growth conditions of 2D NSs is challenging.
2.2. Beyond solution-based chemical approaches
Because the solution-based approaches suffer from the aforementioned limitations, some facile and environmentally friendly methods including cathodic exfoliation and mechanical approaches have been developed, providing new possibilities for the preparation of 2DMNSs.
2.2.1. Cathodic exfoliation method
It has been reported that exfoliated 2D NSs of layered metal chalcogenides and Aurivillius-type layered metal oxides can be obtained by the hydrogen evolution reaction (HER) with the help of intercalated Li+ in the interlayer space [76, 77]. Specifically, the Li+ ion inserted in gap of layers is positioned. When deionized water was added to Li-intercalated powder, during the reaction with H2O, the lithium ion was extracted into solution as LiOH during the reaction with H2O. This process is usually accompanied by hydrogen evolution, which causes drastic volume expansion of the host lattice and subsequent physical separation of the bulk into individual NSs. Via a similar mechanism, Li's group [66] developed a cathodic exfoliation method to obtain ultrathin 2D Sb NSs by exploiting the unique rhombohedral layered structure of the Sb crystal (figures 5(a)–(c)). Specifically, the exfoliation was carried out at a direct current potential of 10 V in a two-electrode system with 0.5 M Na2SO4 as the electrolyte by connecting a pure Sb crystal a copper wire cathode and a Pt foil anode. Consequently, few-layer Sb NSs with an average lateral size of 350 nm and thickness of ∼3.5 nm were obtained from the bulk crystal. Moreover, when the anodic Pt was replaced with graphite, a Sb NS-graphene composite was formed in situ. Although cathodic exfoliation is an efficient and environmentally friendly method to obtain Sb NSs, by exploiting the essential nature of Sb, it is not a universal strategy because it cannot be applied to metals without layered structures.
Figure 5. (a) Schematic of the electrochemical cell for the preparation of the Sb nanosheet-graphene composite. (b) Side-view of the crystal structure of Sb and schematic illustration of the electrochemical exfoliation procedure for Sb nanosheet preparation. (c) TEM image of Sb nanosheets. (d) Schematic of fabrication of metal nanosheets by the calendering method. (e) SEM and AFM images of Ag nanosheets. (f) Schematic illustration of fabrication of Bi nanosheets via hot-pressing. (g) FESEM image and thickness distribution graph of Bi nanosheets. (a)–(c) [66] John Wiley & Sons, © 2017 Wiley‐VCH Verlag GmbH & Co. KGaA, Weinheim. (d) and (e) [68] John Wiley & Sons, © 2016 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim. (f) and (g) [67] John Wiley & Sons, © 2017 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.
Download figure:
Standard image High-resolution image2.2.2. Mechanical approaches
Because of their excellent malleability, most metals can specifically endure considerable plastic deformation; therefore, via mechanical pressing, it should be possible to directly obtain ultrathin metal NSs from bulk metal. On this basis, facile and effective mechanical approaches have been newly developed. Inspired by the traditional Chinese food 'thousand-layer pie' and the malleability feature of metals, Wu's group [68] developed a unique fabrication method, which can be applied to various kinds of metals. First, two heterogeneous metal foils were stacked, followed by repeated folding and rolling for about 20 times at room temperature with a calender machine. Consequently, a layered structure, in which two kinds of metals were alternately stacked, was formed; the thickness of each metal layer was around a few nanometers. Finally, after the selective etching of the same type of metal layers, the 2DMNSs were collected (figures 5(d) and (e)). Via this method, highly separated Ag NSs were prepared by dissolving Al layers of the Al/Ag layered bulk metal in alkaline solutions. The thicknesses of the Ag NSs were in the range 1–10 nm and the lateral sizes were approximately 3–5 μm. Similarly, Au, Fe, Cu, and Ni NSs were also prepared. Although this method is suitable for several types of metals because of their ductile nature, it suffers from the limitation that the ductility of the sacrificial and target metals should be similar, otherwise, the 'thousand-layer pie' cannot be obtained because the harder metal will break during calendering.
Similarly, they also prepared bismuth NSs on highly polished silicon substrates using pure Bi nanoparticles by a facile method [67]. First, 50 μl of Bi nanoparticle dispersion (1 mg Bi dispersed in 25 ml ethanol) was dropped on a silicon substrate. Next, another silicon substrate was placed on the top, and then, the pair was placed in the middle of steel plates of a hot-press machine. During hot-pressing, the pressure was increased up to 0.54 GPa, and the silicon substrates were pressed for 30 min at 150 °C (figures 5(f) and (g)). The thickness of the fabricated Bi NSs ranged from 2 to 10 nm, indicating their 2D structure.
Mechanical approach has opened the doors of a 'Brave New World' in the field of 2DMNSs. In contrast to other methods, it is more facile, effective, universal, and eco-friendly; moreover, it can be used for the synthesis of NSs of various metals as well as non-layered materials, as long as the bulk can endure considerable plastic deformation. Moreover, the high quality and individual characteristics of the final product enables its extensively application, either directly as a function material or as a template in further chemical synthesis. Although the strategy is unsuitable for highly reactive or hard metals (alkali metals or tungsten), it is considered a potential method for the large-scale preparation of 2DMNSs.
3. Applications
Because thicknesses of 2DMNSs are in the atomic range, the exposed constitute the majority of the total atoms, resulting in numerous active sites on the 2DMNS surface. As the physical and chemical environments of surface atoms are different from those of the inner atoms, 2DMNSs possess unique properties, which are absent in the bulk counterpart. Although, only a few 2DMNSs have been prepared, their applications are not properly explored. Nonetheless, they have been confirmed to have potential application in diverse fields including catalysis, optics, chemical cells, and biomedicine. Here, we have summarized the applications of 2DMNSs in catalysis, optics, and biomedicine.
3.1. Catalysis
Because the catalytically active sites are mainly located on the surfaces or edges of catalysts, the presence of a high proportion of exposed atoms in 2DMNSs renders these materials with the maximum atom utilization efficiency, which causes a reduction in the cost of raw materials and enhancement in the catalytic efficiency.
3.1.1. Oxygen reduction reaction (ORR)
Reportedly, 2DMNSs have exhibited excellent catalytic performances in various catalytic reactions in the field of fuel cells. Typically, it is known that it is of great significance to promote ORR in developing proton exchange membrane (PEM) fuel cells owing to their high energy conversion and low pollution. Generally, Pt-based catalysts perform well in facilitating the ORR, but Pt is too expensive to be used in commercial PEM fuel cells. Therefore, the development of high performance catalysts containing low amounts of Pt is essential. For instance, 2D Pt-based catalysts are considered the ideal candidates.
Pt NSs obtained from ultrathin Pt/Ag NSs exhibited excellent electrocatalytic activity toward the ORR in the potential range 0.05–1.03 V (versus RHE) in 0.1 M O2-saturated HClO4. Owing to their ultrathin thicknesses, provision of favorable crystal facets, abundant structural defects, and presence of residuary Ag, the specific activity and mass activity of the Pt NSs were respectively 22 and 9.5 times higher than those of the commercial Pt/C catalyst. Moreover, the electrochemically active surface area (ECSA) of the Pt NSs only dropped by 17.0% after 10 000 cycles of potential sweeps, while that of the commercial Pt/C decreased by 63.6% [56].
PtPb bimetal NSs also showed high specific activity, mass activity, and significantly enhanced catalytic stability in the ORR in an O2-saturated 0.1 M HClO4 solution. The specific activity of PtPb NSs/C reached 7.8 mA cm−2 at 0.9 V (versus RHE), which was 4.1 and 33.9 times greater than those of PtPb nanoparticles/C and commercial Pt/C, respectively. In addition, the mass activity of PtPb nanoplates/C was 4.3 A mg−1 Pt at 0.9 V (versus RHE), which was ∼9.8 times higher than that of the target set by the US Department of Energy for 2020. To investigate the superior performance, DFT calculations were performed, and the results revealed that the edge-Pt and top (bottom)-Pt(110) facets underwent large tensile strains, which optimized the Pt–O bond strength and enhanced the catalytic stability in the ORR. The electrochemical stability of the NSs was evaluated in the potential range 0.6–1.1 V (versus RHE) in 0.1 M HClO4 solution. After 50 000 sweeping cycles, no shift was observed in the ORR polarization curves and only 7.7% loss of mass activity was detected, which was attributed to the intermetallic core and uniform four layers of Pt shell of the NSs. In addition, the PtPb NSs/C also exhibited high electrocatalytic activity and stability toward anodic fuel cell reactions such as the methanol oxidation reaction (MOR) and ethanol oxidation reaction (EOR). The MOR mass activity was 1.5 A mg−1 Pt, which was 2.4 times and 7.9 times higher than of PtPb nanoparticles and Pt catalysts, respectively. Moreover, the EOR specific activity was 2.5 mA cm−2 and mass activity was 1.4 A mg−1 Pt, which were respectively 1.9 and 2.5 times higher than those of PtPb nanoparticles/C, and 10.4 times and 8.8 times higher than those of commercial Pt/C [70]. By carrying out first principle calculations on free-standing Pt-monolayer NSs, Mahata and coworkers [78] predicted and confirmed that the 2D NSs acted as powerful catalysts for the efficient and selective reduction of O2. The results indicated that in the presence of Pt-monolayer NSSs, the ORR became more thermodynamically favorable, and the reaction kinetics improved in the water medium.
3.1.2. Formic acid oxidation (FAO)
In addition to efficiently facilitating the ORR, some 2DMNSs have been reported effective in promoting FAO. FAO is a crucial reaction taking place in formic acid fuel cells, which are considered potential sustainable power sources because they provide clean and efficient energy [79]. Pd-based electrocatalysts are reported to be good candidates for FAO because of their high power densities and remarkable anti-poisoning capacities [79]. However, similar to that of Pt, the wide application of Pd-based electrocatalysts is limited by their high costs.
Toward electrocatalytic FAO in a solution containing 0.5 M H2SO4 and 0.25 M HCOOH, ethylenediamine-treated PdCu alloy NSs [59] exhibited excellent mass activities as high as 1655.7 ± 74.6 mA mg−1 Pd, which were much higher than those of commercial Pd black and other Pd-based catalysts under similar conditions. The superior properties were attributed to the existence of ethylenediamine, which acted as an electron donor and made the PdCu alloy NSs surfaces electron rich, facilitating the adsorption of electron-deficient reactants. Free-standing porous Pd NSs [62] also exhibited high electrocatalytic performance toward FAO, showing much lower onset oxidation potential, higher catalytic activity, and stability than commercial Pd black catalysts. In a solution containing 0.5 M H2SO4 and 0.5 M HCOOH, the onset oxidation potential and peak potential for FAO on 2D porous Pd NSs negatively shifted by about 15 and 64 mV, respectively. In addition, the mass-normalized peak current density of the Pd NSs was 409.3 A g−1, which was two times higher than that of Pd black. After 1000 cycles, for the porous Pd NSs, the ECSA loss was only about 16%, and 57% of the initial oxidation peak current was retained; on the other hand, for commercial Pd black, the ECSA loss was 89% and only 19% of the initial oxidation peak current was retained.
3.1.3. CO2 reduction reaction
Because of the extensive consumption of fossil fuels, CO2 in atmosphere has substantially increased, and therefore, alternative energy sources are in high demand. The conversion of CO2 into formic acid and fuel precursors, such as CO, is a green and economically viable energy technology. Through such a strategy, a sustainable recycling system that reduces the accumulation of CO2 in the atmosphere and produces renewable energy can be achieved. 2D Sb and Cu NSs have been reported to exhibit high performance in the field of CO2 reduction reaction (CO2RR). Specifically, Sb-graphene NSs [66] showed high catalytic capability for the electroreduction of CO2 to formate; a Tafel slope of 110 mV dec–1 was obtained in CO2-saturated 0.5 M NaHCO3 solution. In contrast, bulk Sb was found to be inactive toward the reaction. Owing to high surface area of the hybrid Cu/Ni(OH)2 NSs [69] and promising electrocatalytic property of Cu-based catalysts for the CO2 reduction, the Cu/Ni(OH)2 NSs exhibited an excellent catalytic performance in the selective electroreduction of CO2 into CO at low overpotentials. Interestingly, the use of Cu/Ni(OH)2 NSs enabled the direct production of tunable syngas from CO2 and water at different reduction potentials between −0.4 V and −1.0 V in a 0.5 M NaHCO3 solution. The maximum Faradaic efficiency of 92% toward CO was achieved at −0.5 V with an overpotential of 0.39 V, and the current density was determined to be 4.3 mA cm−2. In addition, the Cu/Ni(OH)2 NSs exhibited no current decay for more than 22 h, indicating the high stability of the catalyst.
3.1.4. Other catalytic applications
Although, 2DMNSs are reported to be effective catalysts for the oxidation of CO, HER, and hydrogenation of phenol, relevant reports are very few. CO methanation involves the direct hydrogenation of CO to CH4 with the consumption of H2. This process is regarded a less costly substitute for the production of H2 from syngas via the preferential oxidation of CO using fuel cells. In addition, CO selectivity has been determined by comparing the conversion ratios of CO and CH4 species. Ultrathin Ru NSs [60] have been employed as catalysts for selective CO methanation reactions. They showed significantly higher CO selectivity than spherical nanocrystals, while maintaining the residual CO concentrations below 10 ppm at temperatures between 204 °C and 250 °C. The concentrations of outlet CH4 at 214 °C and 250 °C were respectively 56% and 22%. However, the detailed catalytic mechanism of these Ru nanocrystals were not revealed [80].
Because Pt-based materials usually perform well in the HER, PtAgCo NSs [61] were prepared for the HER. They showed high catalytic efficiency in N2-saturated 0.5 M H2SO4 solution. Moreover, carbon-supported NSs with 0.01 mmol concentration of Co exhibited the highest activity toward the HER with a large current density of 705 mA cm−2 at a potential of −400 mV and a low Tafel slope of 27 mV dec−1, both of which were higher than those of commercial 20% and 40% Pt/C. The alloying of Pt with Co atoms led to downshift in the d-band centers of Pt with respect to the Fermi levels, which facilitated the HER process by decreasing the adsorption energy of H+ on Pt.
Rh NSs [65] exhibited excellent performance as catalysts in hydrogenation of phenol, resulting in >99.9% conversion within 4 h at near-room temperature under low H2 pressure. Their catalytic activity was four times greater than that of commercial Rh/C catalysts. In addition, in the hydroformylation of 1-octene, the Rh NSs performed better than commercial Rh/C catalysts. The superior performance of was attributed to the high proportion of surface Rh atoms in the Rh NSs, a unique feature of 2D materials.
In short, the development of new energy (H2 and CH4) systems and efficient fuel cells is regarded as a promising approach to meet the energy requirements of the future. In this respect, the role of 2DMNSs, which are confirmed to be efficient catalysts, is highly significant. The catalytic studies performed using 2DMNSs can be used as a reference to further expand the application of 2DMNSs. We believe that the studies being performed on 2DMNSs in the fields of energy and catalysis will help to widen the scope of application of the materials.
3.2. Optical and biomedical applications
In some molecules, the electromagnetic field can be enhanced and confined by coupling the incident light with the resonant motion of the free electron plasma; the phenomenon is called SPR. Accordingly, such plasmonic materials can be used as sensors to detect chemical and biological agents through surface-enhanced Raman scattering (SERS) or near-field optical probing [81–83]. In addition, the SPR properties make them promising materials for biomedical applications such as photothermal cancer therapy [64, 75] and bio-imaging. It has been confirmed that the SPR of nanomaterials is highly sensitive to size and morphology, nanocrystals with shape edges or corners. They exhibited enhanced SPR properties owing to the stronger field enhancement near their shape surfaces [84, 85].
Since 2D NSs are determined to have more shape edges than bulk in the same volume, the SPR properties could be greatly improved and bring about correlative application. In 2012, Yin and coworkers [60] detected stronger SERS signals from Ru NSs with sharp edges and corners gave as compared to the relatively smooth spherical Ru nanoparticles and bulk. In 2018, Zhang's group [74] prepared Au@AuAg yolk-shell triangular NSs with controlled interior gap; the NSs showed SERS of rhodamine 6 G with an enhancement factor of 3.2 × 106. Through these studies, promising candidates for SERS-based trace detection could be realized, providing guidelines for the design and synthesis of 2D NSs with enhanced plasmonic properties.
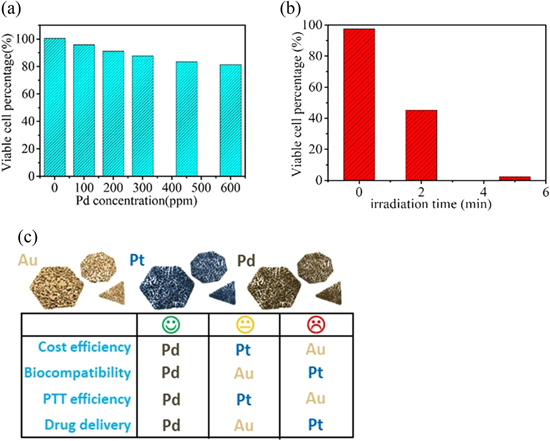
Huang's group [64] reported that 2D palladium NSs exhibited strong SPR absorption in the near-infrared region, which could be used for cancer therapy. More importantly, the NSs exhibited higher photothermal stability and biocompatibility than Ag and Au. Even after irradiation for 30 min with a 2 W, 808 nm laser, the NSs retain their sheet-like structure. In addition, the NSs exhibited biocompatibility: the viable cell count for healthy liver cells was reduced by only 20% after 48 h of exposure in a 600 μg ml−1 solution of Pd NSs (figure 6(a)). Moreover, 100% of the human hepatoma cells were destroyed after 5 min of irradiation with an 808 nm laser providing 1.4 W cm−2 (figure 6(b)). These features make Pd NSs promising candidates for cancer photothermal killing using near-infrared lasers, extending the application of 2DMNSs to the biochemical field.
Figure 6. (a) Viability of healthy liver cells incubated for 48 h with different concentrations of palladium nanosheets. (b) Viability of human hepatoma cells upon irradiation using an 808 nm laser with a power density of 1.4 W cm−2 for various periods. Before irradiation, the cells were incubated with palladium nanosheets (20 μg ml−1) for 12 h. Cell viabilities were measured by the standard MTT assay. (c) Comparison of properties of Pd, Au, and Pt nanosheets for gene-thermo combinational therapy. (a) and (b) [64] Copyright © 2010, with permissions of Springer Nature. (c) Reprinted with permission from [75]. Copyright (2010) American Chemical Society.
Download figure:
Standard image High-resolution imageMore in-depth studies have been performed. For instance, the first report of a quantitative comparison of the biomedical application potentials of Au, Pt, and Pd with the same dimension and morphology (figure 6(c)) was given by Seounghun Kang's group in 2018 [75]. They synthesized porous Au, Pt, and Pd NSs, which exhibited high absorbance in the entire wavelength spectrum, making them promising materials for near-infrared photothermal conversion. The efficacy of gene-thermo combination therapy in cancer was evaluated in NS3-Huh7 cells using MTT cell viability assays. The results showed that the performance of porous Pd NSs was superior in photothermal conversion, therapeutic gene loading/releasing, cytotoxicity, and in vitro combination cancer treatment compared with that of Au and Pt NSs, individually. This discovery may broaden the potential applications of 2D metal nanomaterials and expand the practical applications of Pd NSs.
In biochemical imaging, Ag NSs have been reported to be effective optical contrast agents because of their unique optical properties, which can be tuned by controlling the sheet aspect ratio and morphology [54, 86–89]. In 2012, Homan's group [89] synthesized Ag NSs with tunable thicknesses in the range 10–32 nm. The NSs had a large absorption cross section of light in the near-infrared wavelength region. Cell viability tests showed no statistically significant cytotoxicity at Ag NS concentrations up to 1 mg ml−1. The near-infrared properties, low cytotoxicity, and biological stability of the Ag NSs make them an highly suitable for biomedical imaging and sensing applications.
Table 2. Compilation of representative 2D metal nanosheets.
| Material | Synthesis method | Thickness/nm | Application | References |
|---|---|---|---|---|
| Ag | Conventional wet chemistry | 8–14 | SPR | [54] |
| Au/GO | Conventional wet chemistry, template | 2.4 | — | [55] |
| Cu/Ni(OH)2 | Hydrothermal template | <3 | Electroreduction of CO2 into CO | [69] |
| Pt/Ag | Conventional wet chemistry, template | 1–2 | ORR | [56] |
| Rh | Conventional wet chemistry | 1.3 | — | [57] |
| Rh | Conventional wet chemistry | 7.5–11.0 | — | [63] |
| Pd | Conventional wet chemistry | 1.8 | Cancer therapy | [64] |
| Pd | Conventional wet chemistry | 10 | — | [71] |
| Pd | Conventional wet chemistry | — | — | [72] |
| Pt/Pb/Pt | Conventional wet chemistry, template | 0.8–1.2 | ORR MOR EOR | [70] |
| Pd/Cu | Hydrothermal | 2.8 | FAO | [59] |
| Ru | Hydrothermal | <4, <2 | SERS, CO methanation reaction | [60] |
| PtAgCo | Hydrothermal | — | HER | [61] |
| Pd | Hydrothermal | 10 | FAO | [62] |
| Rh | Solvothermal | <0.4 | hydrogenation of phenol and 1-octene | [65] |
| Sb | Solvothermal | 3.5 | electroreduction of CO2 to formate | [66] |
| Bi | Hot-Pressing | 2–10 | — | [67] |
| Ag | Calendering | 1–10 | — | [68] |
| Pt | Calculation | monolayer | ORR | [78] |
| Au/AuAg | Conventional wet chemistry, template | <10 | SERS | [74] |
| Porous Au, Pt and Pd | Conventional wet chemistry, template | 20 | Gene-thermo combinational therapy | [75] |
| Ag | Conventional wet chemistry | 10–32 | Biochemical imaging | [89] |
4. Summary and outlook
Tuning and controlling the size and the morphologies of metal nanostructures play a very important role in deciding the properties and applications of the nanomaterials. Here, we give an overview of the recent developments in the synthesis of 2DMNSs, as well as their properties and applications (see table 2). 2DMNSs exhibit unique physical and chemical properties because their atomic thicknesses cause the exposure of a large surface area. Meanwhile, the cost could be rapidly reduced by converting metal bulk to ultrathin sheets, which play a critical role in maximum utilization of noble-metal-based catalysts. To date, various methods have been employed to synthesize 2DMNSs, including the typical solution-based chemical approaches, cathodic exfoliation, and the newly developed mechanical approaches. Notably, the typical liquid-phase synthesis has been very effective in producing 2DMNSs; however, the novel mechanical approaches, which exploit the malleability feature of metals, can produce 2DMNSs in a more efficient and environmentally friendly manner. Because of their unique structures, 2DMNSs find potential applications in catalysis, optics, biochemistry, and particularly, fuel cells. 2DMNSs need to be further explored, considering the following aspects:
- (1)Because 2DMNSs are easy to pile up with each other to lower the surface energy and lead to decrease of specific surface area. Correspondingly, it would debilitate performance in various application, new effective methods to stabilize mental NSs are required to be further explored. Selection of proper substrates to stabilize MNSs is a potential strategy. It has been proven that the specific surface area and independence could be guaranteed to reach maximization by loading NSs vertically on substrates densely, which results in great improvements of certain properties [90–92].
- (2)2DMNSs have high surface areas, which make them suitable as platforms for deposition of other materials. Accordingly, combining different types of 2DMNSs is a possible strategy to prepare novel 2D composite materials with desired structures and superior properties.
- (3)Transformation of 2DMNSs either partly or entirely into oxides, phosphides, sulfides, carbides, and nitrides without destroying their unique 2D structures is a promising and effective strategy to produce various types of 2D materials. The resulting materials are expected to possess superior properties, making them suitable for a wide range of applications in various domains.
- (4)As widely reported, doped catalysts exhibit enhanced properties because of the synergistic effect between the native atoms and adjacent doped atoms [40, 93–95]. Therefore, possibly, by anchoring different metals on 2DMNSs or by replacing partial atom in 2DMNSs with other atoms, 2D multimetal NSs with enhanced properties can be obtained. Moreover, introduction of defects or nanoholes into 2DMNSs can enhance the number of active sites, leading to higher catalytic efficiencies.
- (5)The catalytic mechanism of 2DMNSs needs to be further studied by first- principles calculations, which will help to better understand the complicated catalytic processes. In addition, for further modification of 2DMNSs, the influence of dopant atoms on 2DMNSs and their interactions need to be explored at the atomic and molecular levels.
Although some MNSs have been synthesized and investigated, more in-depth studies are required to explore and develop new approaches, which are more facile, efficient, and environmentally friendly, for the preparation of a wide variety of 2DMNSs. Moreover, the scope of application of 2DMNSs need to be widened. We believe that in the near future, intensive efforts will result in the development of various novel 2DMNSs.
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (Grant No. 51 672 194, 51702241, and 51872210), Program for Innovative Teams of Outstanding Young and Middle-aged Researchers in the Higher Education Institutions of Hubei Province (T201602) and Key Program of Natural Science Foundation of Hubei Province, China (Contract No. 2017CFA004).