Abstract
Aluminum nanoparticles are considered promising as alternatives to conventional ink materials, replacing silver and copper nanoparticles, due to their extremely low cost and low melting temperature. However, a serious obstacle to realizing their use as conductive ink materials is the oxidation of aluminum. In this research, we synthesized the oxide-free aluminum nanoparticles using catalytic decomposition and an oleic acid coating method, and these materials were applied to conductive ink for the first time. The injection time of oleic acid determines the size of the aluminum nanoparticles by forming a self-assembled monolayer on the nanoparticles instead of allowing the formation of an oxide phase. Fabricated nanoparticles were analyzed by transmission electron microscopy and x-ray photoelectron spectroscopy to verify their structural and chemical composition. In addition, conductive inks made of these nanoparticles exhibit electrical properties when they are sintered at over 300 °C in a reducing atmosphere. This result shows that aluminum nanoparticles can be used as an alternative conductive material in printed electronics and can solve the cost issues associated with noble metals.
Export citation and abstract BibTeX RIS
1. Introduction
Conventional electronic devices are fabricated using lithography, which is a complex, time-consuming multiple-step process. In addition, this process creates environmental problems because it generates hazardous waste. For these reasons, inkjet printing technology is attractive for its simple processing, low cost, and mass production capability [1]. Moreover, this process enables environmentally friendly processing by reducing energy consumption and the use of chemical pollutants [2]. As a result, inkjet printing has been considered for manufacturing applications such as printing radio-frequency identification (RFID) tags [3], organic thin-film transistors (OTFTs) [4], organic light-emitting diodes (OLEDs) [5], and organic photovoltaic (OPV) devices [6], which require mass production technologies to reduce their costs. To manufacture electronic devices, the development of conductive ink is essential. Many conductive materials, such as conducting polymers [7], carbon-based materials [8, 9] and metal nanoparticles [10, 11] have been studied as conductive inks. From this research, metal nanoparticles are regarded as the best candidates for conductive inks due to their higher electrical conductivity and simpler syntheses than those of conducting polymers (102–103 S cm−1) and carbon-based materials (10–102 S cm−1), which have relative low conductivity.
At present, most metal nanoparticle inks are based on noble metals such as silver and gold because of their high electrical conductivity and stability in air. Although considerable research on noble metal nanoparticles has been done and their inks are already commercially available, efforts are underway to replace them due to their extremely high cost. Other conductive nanoparticles fabricated from metals such as aluminum [12], copper [11], tin [13] and nickel [14] have been studied as alternative materials. In particular, aluminum nanoparticles are considered to be a candidate for novel conductive inks because they have higher electrical conductivity than silver nanoparticles and are made from the least expensive conductive metal. In addition, aluminum nanoparticles can be used as the rear electrode in OPV or OLED application [15, 16] due to their low work function compared to noble metals [17].
Although their theoretical properties are suitable for conductive materials, aluminum nanoparticles have not been studied in connection to conductive inks because they suffer from a serious drawback; the formation of a thermodynamically stable surface oxide layer [18]. Until now, the native oxide of aluminum has been negligible (3 ∼ 5 nm) when used in bulk or on the microscale. However, when using aluminum nanoparticles as a component of conductive ink in printed electronics, the presence of this oxide can significantly degrade their electronic performance due to their high surface-to-volume ratio. Many studies have been performed in the case of copper nanoparticles, which also have severe oxidation problems. Surface coatings using other materials such as carbon, silica, organic surfactants and second metals have been investigated as a solution [19–25]. Among these, the method of coating the nanoparticles with organic surfactants has the advantages of a stable dispersion in the solvent and long-term chemical stability. The organic coating method is suitable for aluminum nanoparticles due to their high reactivity. In addition, several studies have been reported, although not for the same applications, which pertain to retaining the active surface of aluminum using organic material coatings for use with fuel or rocket propellants [26, 27].
In this study, we used a simple catalytic decomposition process to synthesize oxide-free aluminum nanoparticles capped with oleic acid. We demonstrated the synthetic mechanism of aluminum nanoparticles and their stability in air with a self-assembled monolayer (SAM) of oleic acid. Through an x-ray photoelectron spectroscopy (XPS) analysis, the chemical structure of nanoparticles with complete surface capping by oleic acid was determined. Then, to confirm the potential for using alternative materials in printed electronics, we performed a simple printing process by spin coating aluminum nano-ink onto a glass substrate. The fabricated conductive films were sintered under different conditions, and their electrical properties were measured using a four-point probe station.
2. Experimental details
2.1. Preparation of oxide-free Al nanoparticles
A simple solution process was used to synthesize oxide-free Al nanoparticles. Alane N,N-dimethylethylamine complex solution (0.5M in toluene, Sigma-Aldrich) was used as the Al precursor and Ti(IV) isopropoxide (99.999%, Sigma-Aldrich) was used as the catalyst. Tetrahydrofuran (THF, anhydrous, Sigma-Aldrich) was used as the solvent and washing agent. Oleic acid (OA, technical grade, 90%, Sigma-Aldrich) was used as the capping agent, which acted as a stabilizer and anti-oxidant. All the reagents were used as received, without further processing or purification, and they were treated under an argon atmosphere in a glove box to prevent oxidation. To synthesize the Al nanoparticles, we used the catalytic decomposition method invented by Haber and Buhro [28]. 5 ml of alane precursor was prepared in a vial and 4.4 μl of Ti catalyst (0.6 mol% of alane precursor) was gently added to the vial whilst being vigorously stirred with a magnetic bar at room temperature. The solution abruptly changed color to dark brown with the evolution of a gas. It then gradually changed color to black, indicating the formation of Al nanoparticles. After 1 h, we injected 20 ml of tetrahydrofuran to wash out the reacting residue. Pure Al nanoparticles were obtained after 30 min of centrifugation at 10 000 rpm. The mixture of tetrahydrofuran and 0.316 ml of oleic acid (0.5 mol of Al) was then injected into the Al nanoparticles to prevent oxidation and growth. We had now obtained oxide-free oleic acid capped Al nanoparticles.
2.2. Preparation of Al nano-ink and conductive film
To fabricate aluminum nano-ink, the as-synthesized oxide-free aluminum nanoparticles needed to undergo a washing process to remove excess oleic acid. After the nanoparticles, along with the mixture of toluene and ethanol, were centrifuged at 10 000 rpm for 30 min several times, the remaining nanoparticles were dispersed into the toluene solution with a particle content of 30 wt%. The prepared nano-ink then homogenized on the rolling station overnight. Conductive film was fabricated by spin coating aluminum nano-ink onto glass substrate. The spin coating was conducted in a glove box in an argon atmosphere to prevent surface oxidation and the speed of spin coating was 1000 rpm, for 30 s. After this it was sintered in a reducing atmosphere (10% hydrogen with 90% argon gas) at temperatures of 200 °C, 300 °C, 400 °C, 500 °C and 600 °C. To measure the electrical resistivity of the conductive film, we needed data on two factors: sheet resistance and the thickness of the conductive film. The sheet resistance was measured using a four-point probe. The thickness was measured using scanning electron microscopy (SEM) analysis by obtaining an image of a vertical section of the conductive film.
2.3. Characterization
The size and shape of Al nanoparticles were observed by transmission electron microscopy (TEM, JEM-2100F, JEOL) and high-resolution TEM (HRTEM, F30 ST, Tecnai). Selected area electron diffraction (SAED) was used to determine the microstructure of the Al nanoparticles and the presence of a uniform organic layer. To calculate the size distribution of the Al nanoparticles, we used TEM images to select more than 100 nanoparticles. We prepared samples for the TEM analysis by dropping tetrahydrofuran solution containing Al nanoparticles onto copper grids coated with carbon film. To confirm the existence of an organic layer around the nanoparticles, we used x-ray photoelectron spectroscopy. Oleic acid capped Al nanoparticles were dried in vacuum and placed on a wafer substrate for XPS analysis, which was used to determine the binding energy between the organic ligand and the metal surface. The prepared nanoparticles were stored in a low humidity desiccator for two days before XPS measurements, which means that the oxidation stability of nanoparticles in this study is limited to two days in air. The values of the binding energy were calibrated using the C 1s peak, which was set at 284.5 eV. To analyze the surface morphology and the thickness of the film after sintering, we used images from a field emission scanning electron microscope (FESEM, Nova230). To characterize the decomposing temperature of the capping molecule, we conducted thermal gravimetric analysis (TGA) (Mettler–Toledo), where the temperature was increased from 25 °C to 700 °C at a heating rate of 10 °C min−1 with the sample under a nitrogen atmosphere. To determine the structure of Al film after sintering, x-ray diffraction (XRD) data were obtained using a Rigaku D/max-2500 diffractometer with Cu Kα radiation at a scan rate of 1 ° min−1. A four-point probe (CMT-SR1000N) was used to measure the sheet resistance. The contact potential difference was measured using a scanning Kelvin probe microscope (SKPM, Park Systems).
3. Results and discussion
3.1. Synthetic mechanism and size control of Al nanoparticles
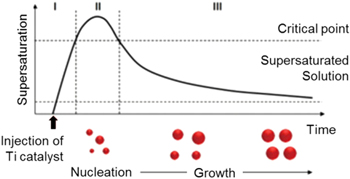
The oxide-free aluminum nanoparticles were synthesized by catalytic decomposition of an alane precursor in the presence of oleic acid, which prevents the formation of the surface oxide of aluminum nanoparticles. The size of the aluminum nanoparticles was varied by the injection time of oleic acid. This synthetic mechanism can be explained by figure 1, which shows the nucleation and growth of aluminum nanoparticles with a La Mer plot [29]. When the catalyst was injected ino the alane precursor, the burst nucleation of aluminum nanoparticles took place at the critical point of supersaturation. The growth step followed, and the size of the aluminum nanoparticles increased through ripening other particles with the passage of time. Therefore, aluminum nanoparticles were grown by 22.3 nm, 40.1 nm and 169.53 nm, respectively, over the time periods of 1 h, 2 h and 3 h, as shown in figure 2. The completion of the growth step was closely related to the injection of oleic acid because the functional group of carboxylic acid was forming a SAM on the aluminum surface. The mechanism of the formation of a SAM of carboxylic acid onto aluminum nanoparticles was reported by Crowell et al [30], and the close packing of the organic layer effectively protects the nanoparticles from oxidation [31].
Figure 1. Synthetic mechanism of nanoparticles shown by La Mer plot. (Stage I) supersaturation of monomer, (stage II) nucleation stage when monomer concentration is high enough for nucleation, (stage III) time dependent growth stage.
Download figure:
Standard image High-resolution imageFigure 2. Size control of Al nanoparticles by controlling growth time. We injected surface capping agent after (a) 1 h (22.3 nm), (b) 2 h (40.1 nm), (c) 3 h (169.5 nm).
Download figure:
Standard image High-resolution image3.2. Structural analysis of SAM-modified Al nanoparticles by carboxylic acid
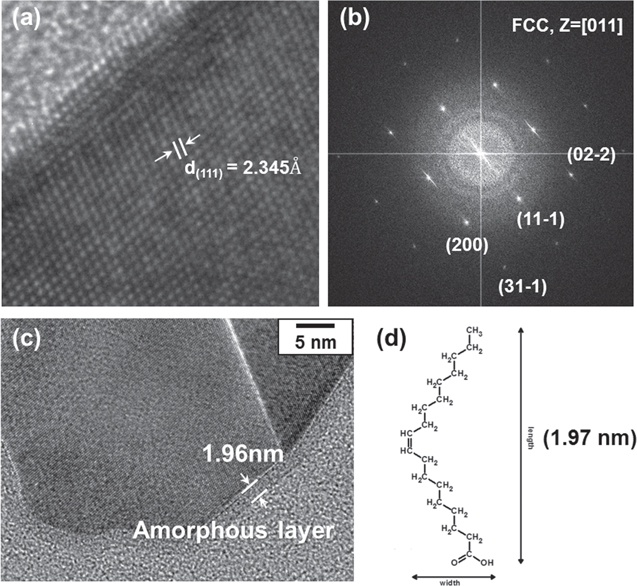
The SAED and HRTEM images in figures 3(a), (b) were used to confirm the microstructure of aluminum nanoparticles. A clear and continuous lattice fringe was observed, and the interplanar distances (0.24 nm) measured on the images corresponded to the aluminum orientation of FCC (111) planes, which is also in agreement with the SAED pattern. As shown in figure 3(c), crystalline aluminum nanoparticles were surrounded by a uniform 1.96 nm amorphous layer, which are considered to be capping the molecule. Although the thickness of the amorphous layer was the length of the oleic acid monomer, it was not fully demonstrated that oleic acid surrounds the aluminum nanoparticles.
Figure 3. Structural analysis of Al nanoparticles and their surface. (a) HRTEM image of crystalline Al nanoparticles (lattice constant is matched with (111) surface of Al), (b) SAED pattern of Al nanoparticles (indexing of diffraction pattern indicates that structure of Al is FCC), (c) organic amorphous layer exists on the surface of Al nanoparticles, (d) length of oleic acid monomer.
Download figure:
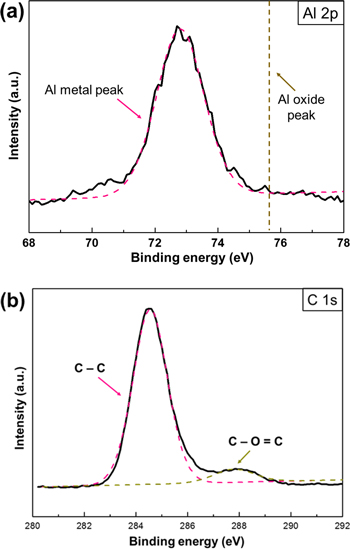
Standard image High-resolution imageTo precisely identify the amorphous layer, XPS analysis was carried out. In the case of the oleic acid capped aluminum nanoparticles we carefully observed the two main spectra, Al 2p and C 1s. As shown in figure 4(b), two C 1s peaks were observed at 284.5 eV and 288.4 eV. Generally, the main peak at 284.5 eV was attributed to the carbon atoms in the alkyl chain (C-C), which indicates the long carbon chain of oleic acid (C18). The shoulder peak at 288.4 eV is assigned to the carboxylate (−COO−) carbon, which corresponds to the other report that the carboxyl functional group was adsorbed on the aluminum surface as a reduction of the hydrogen cation [32]. In addition, the carboxylic carbon peak, which is located at 290.0 eV, did not appear on the C 1s spectrum. Therefore, we confirmed that oleic acid existed around the aluminum nanoparticles with the formation of SAM between the aluminum metal and the carboxylate of oleic acid. On the other hand, the Al 2p peak shows the simple peak at 72.7 eV, which indicates pure aluminum metal bonding. In addition, no oxide peak (75.7 eV) appears in this spectrum, so the XPS results show that aluminum nanoparticles did not have an oxide layer, only an organic capping layer.
Figure 4. Surface analysis of Al nanoparticles with organic capping layer by XPS. (a) Al 2p peak, (b) C 1s peak.
Download figure:
Standard image High-resolution image3.3. Stability and electrical properties of Al film after thermal sintering
In this study, we demonstrate that unoxidized Al nanoparticles are an alternative material for use in conductive electronics printing. The conventional materials used for printing are noble metals which are very costly. With the Al nanoparticle, conductive electronics printing can be done cost effectively. To confirm the characteristics which make it suitable for application in electronics, we prepared conductive film by spin coating with Al nanoparticle ink. The as-prepared Al film is composed of individually isolated particles, which are converted into a continuous film by thermal sintering conducted in a tube furnace in a reducing atmosphere. We then measured the variations in the resistivity of the Al nanoparticle film using a four-point probe. Figure 5 shows electrical resistances in relation to the sintering temperatures. It implies that the electrical resistance of the film is closely related to the temperature at which it is sintered.
Figure 5. Electrical resistivity change by varying sintering temperature, and comparison of electrical properties with related work. Also shown is the fabrication of Al film and its thickness (inset).
Download figure:
Standard image High-resolution imageThe morphological change of the particles in the sintering process is the result of heating causing atom movement. At higher temperatures, atoms have the energy to move to a new position; energy is needed to break the atomic bond and free the atom from neighboring atoms. The minimum energy required for an atom to move is called the activation energy. The number of atoms with sufficient energy to move at high temperatures is explained by a statistical concept derived from the Arrhenius temperature relationship, using the equation below [33].
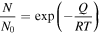
N/N0 is the ratio of moving atoms to all atoms, Q is the activation energy, R is the gas constant and T is temperature. Activation energy is the minimum energy required to free an atom from its neighboring atoms. With higher temperature, more energy is provided to overcome the activation energy. As a result, atoms are more likely to migrate. Therefore, with a higher sintering temperature, the Al nanoparticles are more likely to transform into continuous film.
The transformation of the particles into continuous film was observed using SEM. As shown in figures 6(a)–(c), the surface morphologies of the films from different sintering temperatures are captured. The coalescence starts from 400 °C, which is higher than the boiling temperature of oleic acid (360 °C). When the nanoparticles sintered at 200 °C, the Al nanoparticles exhibited a small particle size, and did not physically contact each other. When the Al nanoparticles sintered at over 400 °C they showed little aggregation between particles and their average size gradually increased as the sintering temperature increased. When they were sintered at 600 °C, the Al nanoparticles were fully in contact with each other because the temperature was elevated to near the melting temperature. Thus, Al nanoparticles show the lowest resistivity (4.12 × 10−5 Ω · cm), which is about ten times greater than that of bulk Al, and we expect that the resistivity will converge to bulk conductivity with sintering at the melting temperature (660 °C). This value is meaningful because its electrical properties have been shown to improve by about two orders of log scale compared with another study [34], and the study of Al nanoparticles for application as a conductive material has not yet been much reported.
Figure 6. Series of surface morphology of Al film after sintering at (a) 200 °C, (b) 400 °C, (c) 600 °C.
Download figure:
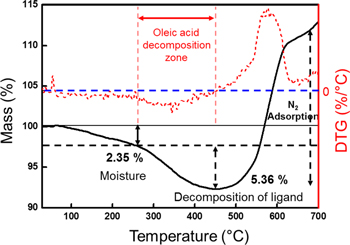
Standard image High-resolution imageThe decomposition of the surface ligand was confirmed by TGA analysis in figure 7. The TGA can be roughly divided into three sections. There are two weight loss sections: decomposition of moisture and decomposition of oleic acid. One is the weight gain section, which is the adsorption of N2. Generally, the moisture or residual solvent was decomposed from the beginning of the TGA, and 2.35% of the moisture decomposed at the first weight loss step. DTG (differential of TG) was used to determine the unclear point of the oleic acid decomposition step. The decomposition of the surface ligand started from 260 °C and the amount of decomposed ligand was 5.36% as a total. However, the slope started to increase at around 400 °C plus, which implies that the organic layer decomposes and gas adsorption occurs at the same time from this point. Therefore, Al nanoparticles can be oxidized without an organic capping layer, but it is stable with an organic layer.
Figure 7. TGA (black) and DTG (red) analysis of Al nanoparticles: the temperature was increased from 25 °C to 700 °C at a heating rate of 10 °C min−1 with the sample under a nitrogen atmosphere.
Download figure:
Standard image High-resolution imageAs mentioned above, the level of oxidation of Al nanoparticles also needs to be considered while sintering. When the surface ligand around the nanoparticles starts to decompose, Al nanoparticles would absorb oxygen or nitrogen from the surrounding atmosphere due to the high surface energy of the bare nanoparticles. For example, when nanoparticles are sintered in a nitrogen atmosphere, nitrogen is adsorbed onto the surface of the Al during the decomposition of the surface ligand, as result of TGA, in figure 7. However, figure 8 shows that only characteristic diffraction peaks of Al with a face-centered-cubic crystal structure (JCPDS 04-0787) appeared in the Al film after sintering in a reducing atmosphere. On the other hand, a crystalline alumina (Al2O3) peak was not detected. To confirm whether an amorphous oxide layer existed, the XPS analysis was carried out. We confirmed that a 3.5 nm thick amorphous oxide was formed on the surface of the Al film (initially) and it increased to 4.3 nm after 18 months. The thickness was calculated by quantitative analysis of the XPS peak [35]. The oxide formation seems native by nature. To confirm the oxide effect on the conductive film, we measured the electrical resistivity of thin film. Even though the resistivity slightly increased to 3.2 × 10−3 Ω · cm, the conductive film maintained its electrical resistivity as low as the level of commercial Ag-epoxy conductive paste. This implied that the film properties became metallic during sintering, and that the amorphous native oxide was formed after sintering.
Figure 8. Oxidation stability analysis of Al film. (a) XRD analysis to confirm crystalline Al2O3, XPS analysis to confirm amorphous oxide layer of Al film, (b) as-prepared, (c) 18 months later.
Download figure:
Standard image High-resolution imageFigure 9. Topography and contact potential difference measurement by scanning Kelvin probe microscopy.
Download figure:
Standard image High-resolution imageIn addition, one of the important properties of Al film is its work function, because Al can be applied to the electrode of an active device such as a photovoltaic or light emitting diode. In fabricating an active device, its structure is critical in enhancing efficiency. A metal electrode with a low work function is preferred to injecting many electrons at low potential. To measure the work function of Al nanoparticles, we used contact potential difference (CPD) via SKPM. Generally, SKPM is considered to be a suitable noncontact method to map the two-dimensional distribution of CPD between the tip and a sample. CPD can be converted into the work function of the sample using the following relation.
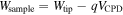
Wsample and Wtip are the work function of the sample and the tip, respectively, q is the electronic charge and VCPD is the CPD measured by SKPM [36]. As shown in figure 9, we already know the work function of the tip (ϕ of PtCr = 4.90 eV) and of the as-prepared substrate (ϕ of ITO = 4.7 eV), so the work function of Al film is 4.22 eV. Al nanoparticles are more efficient than a noble metal such as Ag or Cu for use in the electrode due to its low work function, and the fact that it will improve the efficiency of electron injection in the cathode of an OLED or OPV [37].
4. Conclusion
We synthesized Al nanoparticles using a catalytic decomposition process, and the size of the Al nanoparticles were controlled by the injection of a surface capping agent. In addition, the surface oxidation of Al nanoparticles was successfully controlled using SAM modification of oleic acid. The structure of oxide-free Al nanoparticles was confirmed by TEM and XPS analysis. With these nanoparticles, we fabricated Al film using a simple solution process. Thermal sintering was conducted in a reducing atmosphere to achieve electrical properties. The resulting film exhibited low electrical resistivity and air stability. We achieved a resistivity of 4.12 × 10−5 Ω · cm at a sintering temperature of 600 °C, and we confirmed that the work function of Al nanoparticles was 4.22 eV. These results show that Al nanoparticles can be used to create electrodes for active devices such as OLEDs or OPVs. However, the thermal sintering temperature is still high, so studies should continue with the aim of lowering the sintering temperature, taking into consideration the decomposition temperature of the surface ligand.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science, ICT & Future Planning (MSIP) (No NRF-2015R1A5A1037627).