Abstract
Here, we fabricated polypyrrole nanoparticles (PPys) (termed HA10-PPy, HA20-PPy, and HA40-PPy) doped with different average molecular weight hyaluronic acids (HAs) (10, 20, and 40 kDa, respectively), and evaluated the effect of molecular weight of doped HA on photothermal induction, fluorescence quenching, and drug loading efficiencies. Doxorubicin-loaded HA-doped PPys (DOX@HA–PPys) could be used for imaging and therapy of triple-negative breast cancer (TNBC). Fluorescence turn-on, stimuli-responsive drug release, and photo-induced heating of DOX@HA–PPys enabled not only activatable fluorescence imaging but also subsequent chemo/photothermal dual therapy for TNBC. In particular, we illustrated the potential usefulness of the photothermal effect of the nanoparticles for overcoming chemoresistance in TNBC.
Export citation and abstract BibTeX RIS
1. Introduction
Triple-negative breast cancer (TNBC), which is characterized by a lack of significant expression of the estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 (HER2), is associated with poor prognosis and represents an important clinical challenge [1, 2]. Because of the lack of HER2 and hormone receptor expression, TNBC does not respond to hormonal therapy and HER2-targeted agents, leaving cytotoxic chemotherapy as the only therapeutic option. Doxorubicin (DOX), which is the most commonly used therapeutic individually or in combination with taxane, is considered to be the most effective treatment for TNBC [2–4]. However, despite reasonable response rates to initial chemotherapy in patients with TNBC, most patients with TNBC (63%) have residual disease after long-term treatment with DOX, showing a peak risk of relapse at around 3 years post-treatment, as well as an increased mortality rate (74%) [2, 5–8]. This limited efficacy may be due to de novo drug resistance and/or the development of a drug-resistant cellular phenotype during treatment [8, 9]. Therefore, the development of new therapeutics to overcome the current limitations of TNBC treatment is urgently needed. A good candidate for this may be theranostics, which is the integration of diagnostics and therapeutics in one system, because it has been shown to have potential utility not only in detecting target sites but also in improving treatment outcomes with fewer side effects [10, 11]. Recently, different types of theranostic agents have been tried for fluorescence imaging and therapy of TNBCs [12–14], and development of a biocompatible and highly efficient theranostic agents is an ongoing challenge.
Here, we propose hyaluronic acid (HA)-doped polypyrrole nanoparticles (PPys) with DOX payload (DOX@HA–PPys) as an activatable nanotheranostics for fluorescence imaging with high target-to-background ratio and subsequent chemo/photothermal dual therapy of TNBC. PPys have been considered promising photothermal therapy agents because of their excellent biocompatibility, high molar extinction coefficient in the near-infrared (NIR) region, and strong photothermal effect [15–17]. HA, an anionic glycosaminoglycan with repeating carboxylic acid groups, is abundant in humans, especially in the extracellular matrix, and therefore has been used to improve the biocompatibility of biomaterials [18–20]. In our recent communication article, we demonstrated for the first time a synthetic method for preparing HA–PPys (average molecular weight of 10 kDa) and showed the possibility of using HA–PPys as a nanocarrier and diagnostic agent for macrophages in atherosclerotic lesions [21]. Here, we fabricated PPys (termed HA10-PPy, HA20-PPy, and HA40-PPy) doped with different average molecular weight HAs (10, 20, and 40 kDa, respectively), and evaluated the effect of the molecular weight of doped HA on photothermal induction, fluorescence quenching, and drug loading efficiencies. In the in vitro cell studies, DOX-loaded HA–PPys (i.e., DOX@HA–PPys) showed high photothermal effect, pH- and light-responsive drug delivery, and fluorescence turn-on in TNBC cells with low background signals.
2. Experiment
2.1. Materials
Pyrrole (98%), polyvinylpyrrolidone (PVP, MW ∼29 000), iron (III) chloride hexahydrate (FeCl3·6H2O), and doxorubicin hydrochloride (DOX) were purchased from Sigma-Aldrich (St. Louis, MO, USA). HAs (HA10K, HA20K, and HA40K) were purchased from Lifecore Biomedical (Chaska, MN, USA) and used as received. Microdialysis tubes (D-Tube Dialyzer Mini, molecular weight cutoff (MWCO) 12–14 kDa) were obtained from Novagen (Madison, WI, USA). All other chemicals and solvents were analytical grade and used without further purification.
2.2. Synthesis of PPys and various HA-doped PPys (HA10-PPy, HA20-PPy, and HA40-PPy)
HA–PPys were synthesized using a modified version of a previously published method [21]. Briefly, 400 mg of HA was dissolved in 20 ml of deionized (DI) water. PVP (0.5 g) was dissolved in 12.5 ml DI water with magnetic stirring for 30 min at room temperature (RT), followed by the addition of 65 μl of pyrrole. After 10 min, 0.5 ml of FeCl3·6H2O (0.75 g ml−1) was added to the reaction mixture. HA solution (20 ml) was added simultaneously to the mixture and stirred for 3 h at RT. Unbound HA was removed by dialyzing the solution against DI water using a membrane (MWCO 100 kDa) for 2 days with multiple water exchanges. Large precipitates were removed by centrifugation at 1200 rpm for 2 min, and the supernatant was lyophilized to obtain a black powder.
PPys were prepared with the same protocol described above without adding the HA solution.
2.3. Characterization of PPys and various HA–PPys
To determine the changes in physical characteristics of HA–PPys depending on HA molecular weight, UV/Vis absorption, scanning electron microscopy (SEM), fourier transform-infrared (FT-IR) spectroscopy and thermogravimetric analysis (TGA) were performed. A DU730 UV/Vis spectrophotometer (Beckman Coulter, Brea, CA) was used to measure UV/Vis absorbance of the prepared nanoparticles. Before TGA, all samples were dried in an oven at 80 °C for 2 h. The morphology of PPys and HA–PPys was observed using field emission SEM (JEOL-7001F, JEOL Ltd, Japan).
2.4. Fluorescence quenching of DOX by PPys and HA–PPys
The fluorescence of DOX was monitored upon the addition of increasing amounts of PPys or HA–PPys as quenchers. DOX (100 μl, 0.1 mg ml−1) was mixed with 200 μl of DI water containing various amounts of quencher (0, 10, 20, 40, 60, 80, 100, 120, 150, 200 μg), incubated for 1 h, decanted to a 96-well plate. The fluorescence spectra of DOX at 480 nm excitation wavelength were measured using a Safire 2 multifunctional microplate reader (Tecan, Männedorf, Switzerland). The quenching efficiency was calculated using the Stern–Volmer equation: FO/F (595 nm) = +Ksv [Q]. The X-axis and Y-axis were defined as [Q] (mg l−1) and FO/F (595 nm) −1, respectively.
2.5. Preparation and characterization of DOX-loaded HA40-PPys (DOX@HA40-PPys)
HA40-PPys were dissolved in 400 μl of DI water (10 mg ml−1), and DOX was dissolved in 100 μl DI water to prepare various concentrations (in the case of 1:10 ratio, DOX concentration = 4 mg ml−1). DOX solution was added dropwise to the HA–PPy solution and stirred for 3 h at RT to form DOX@HA–PPys. Free DOX was removed by PD MiniTrap G-25 (GE Healthcare, Uppsala, Sweden) following the manufacturer's protocol, the volume of the eluate was adjusted to 1 ml, and the samples were stored at 4 °C until further use.
The surface charge of the prepared nanoparticles was measured using a zeta potential/particle sizer (Malvern Instrument, Malvern, UK). For the measurement of DOX content of the nanoparticles, DOX@HA40-PPys were dissolved in DI water containing 5% (w/v) sodium dodecyl sulfate (SDS) to release DOX from the nanoparticles by disrupting the electrostatic interaction. The UV/Vis absorbance of the samples at 498 nm was measured and compared with a standard curve generated by using free DOX. For the samples with low UV/Vis absorbance, we could not measure drug content. In these cases, we analyzed fluorescence intensities of the sample solutions 595 nm, and compared them with a standard curve of free DOX.
For the comparison of optical properties, DOX@HA40-PPys were dissolved in DI water, whereas free DOX was dissolved in DI water containing 5% SDS to avoid the unnecessary aggregation and quenching of DOX molecules in neutral pH conditions. UV/Vis absorption and fluorescence spectra were then measured.
2.6. Measurement of temperature increase during light illumination
Nanoparticles dispersed in 1 ml of DI water (0–200 μg ml−1) were prepared in Eppendorf tubes. The light source was an 810 nm CW laser with adjustable power output (6 W 810 nm CW laser, LaserLab, Seoul, Korea). During laser irradiation, the temperature changes of the sample solutions were recorded with a ThermaCAM P25 infrared camera (FLIR, Wilsonville, OR, USA) every 10 s. Data were obtained by calculating the maximum temperature of the irradiated area.
2.7. In vitro drug release profile of DOX from DOX@HA40-PPys
The in vitro release profile of DOX from DOX@HA40-PPys was examined at different pH conditions (pH 7.4 and 5.0). Briefly, DOX@HA40-PPys (150 μl, 80 μM DOX equivalent) were dialyzed using D-Tube Dialyzer Minis (MWCO 12–14 kDa) against 15 ml of phosphate-buffered saline (PBS; 160 mM, pH 7.4, NaCl 150 mM) or acetate buffer (200 mM, pH 5.0) with gentle shaking. Experiments were performed in triplicate.
In DOX release experiments using NIR light irradiation, DOX@HA40-PPy solution was irradiated with an 810 nm CW laser (light dose rate: 1 W cm−2) for 5 min before dialyzing. At each selected time point, 250 μl of buffer was replaced with fresh solution, and fluorescence intensities of the collected samples were measured (λex 480 nm, λem 593 nm).
2.8. Fluorescence intensity change of DOX@HA40-PPys upon light irradiation
DOX@HA40-PPys (200 μl, 10 μM DOX equivalent) were placed in a 96-well plate and fluorescence intensity was checked every 2 min (λex 480 nm, λem 593 nm). The light-treated group was irradiated with an 810 nm CW laser (dose rate: 1 W cm−2) for 5 min repeatedly.
2.9. Cell culture and maintenance
MDA-MB-231 and MDA-MB-231/DOX cells were maintained in RPMI-1640 media supplemented with 10% fetal bovine serum (FBS) and 1% antimicrobials/antimycotics (Life Technology, Gaithersburg, MD, USA) at 37 °C under 5% CO2 in a standard humidified incubator. MDA-MB-231/DOX cells were derived from MDA-MB-231 cells by continuous culture in the presence of DOX for more than three months. Exposure of MDA-MB-231 cells to stepwise increasing concentrations (0.1–2 μM) of DOX resulted in the selection of doxorubicin-resistant MDA-MB-231/DOX cells. To exclude the influence of residual DOX in the cytoplasm, exposure to DOX was terminated 4 days prior to experiments.
2.10. Live cell images of MDA-MB-231 incubated with DOX or DOX@HA40-PPy
Utility of DOX@HA40-PPy in the activatable fluorescence detection of TNBC cells was tested using an in vitro live cell imaging study. MDA-MB-231 cells were seeded onto Nunc 8-well Lab-Tek Chamber Slides (Nalge Nunc International, Penfield, NY, USA) at a density of 2 × 104 cells per well and incubated 48 h. Then, the medium was exchanged with phenol red-free medium containing DOX or DOX@HA40-PPys at 10 μM DOX equivalent. Without washing the medium, fluorescence images of the cells were acquired every 15 min using a live cell imaging system (Axio Observer Z1, 10×, NA 0.55, Carl Zeiss, Oberkochen, Germany). To minimize external factors, all microscopy experiments were performed using identical settings (λex 534–558 nm, λem 575–640 nm) and analyzed with Carl Zeiss software (ZEN lite 2012).
2.11. Biocompatibility evaluation of HA40-PPy
MDA-MB-231 cells were seeded onto 96-well plates at a density of 2 × 104 cells per well and incubated overnight to allow cell attachment. Then, the medium was replaced with fresh media containing various concentrations of HA40-PPys and incubated for 24 h. After washing the cells 2 times with PBS, cell viability was evaluated by a CCK assay according to the manufacturer's protocol (Dojindo Laboratories, Kumamoto, Japan). The viability of the untreated control cells was defined as 100%. Data are expressed as the mean (SD) of four data samples.
2.12. Cytotoxicity assay
First, the chemotherapeutic efficacy of DOX@HA40-PPys was evaluated by treating cells for 24 h in the absence of light illumination and comparing these cells to those treated with free DOX. MDA-MB-231 cells were seeded onto 96-well plates at a density of 1 × 104 cells per well and incubated overnight to allow cell attachment. Both free DOX and DOX@HA40-PPyNP were diluted in fresh DMEM supplemented with 10% FBS to obtain various DOX concentrations. The culture medium was replaced with the fresh cell-culture medium containing free DOX and DOX@HA40-PPyNP; cells were incubated for 24 h, washed twice with cell-culture medium, and further incubated for 44 h to allow sufficient time for the DOX anti-proliferative activity. Cell viability was analyzed using a cell counting kit-8 (CCK-8, Dojindo Laboratories, Kumamoto, Japan). The viability of the untreated control cells was considered 100%.
Next, the potential utility of DOX@HA40-PPys in chemo/photothermal dual therapy was evaluated by treating cells for 4 h. MDA-MB-231 cells were seeded onto 96-well plates at a density of 1 × 104 cells per well and incubated overnight to allow cell attachment. In the free DOX group, the cells were treated with fresh medium containing various concentrations of DOX for 4 h. In the light-treated groups, the cells were treated with fresh medium containing various DOX equivalent concentrations of nanoparticles, irradiated with an 810 nm CW laser (1 W cm−2), followed by incubation for 4 h. For the HA40-PPy plus light group, the concentration of HA40-PPys was adjusted to the HA40-PPy equivalent concentrations in DOX@HA40-PPy solutions. Then, the cells were washed two times with medium and incubated for an additional 44 h. Finally, cell viability was calculated using the CCK-8. Data are expressed as the mean (SD) of four data samples.
2.13. Flow cytometric analysis
MDA-MB-231 cells were seeded onto 12-well plates at a density of 1 × 105 cells per well and incubated overnight to allow cell attachment. After the medium was replaced with fresh medium containing various DOX equivalent concentrations of DOX@HA40-PPys, cells were irradiated with NIR light (810 nm, 1 W cm−2) for 5 min, followed by incubation for 4 h. Then, cells were washed two times with PBS, harvested, and stored in FACS buffer. Data were acquired using a BD FACSVerse (BD Biosciences, Franklin Lakes, NJ, USA) by counting approximately 10 000 events (Ex. 488 nm, Em. 700/54 nm) and analyzed using FlowJo software.
2.14. Cytotoxicity assay of MDA-MB-231/DOX
MDA-MB-231/DOX cells showed different characteristics, including cell size, compared with the original MDA-MB-231 cells. Therefore, we used a modified method in the cytotoxicity assay. A CellTiter-Glo cell proliferation assay (Promega, Madison, WI, USA) was performed using the manufacturer's protocol, with some modifications. Briefly, 5 × 103 cells were seeded in 96-well plates, and after 24 h cells were exposed to DOX individually or DOX@HA40-PPys combined with light treatment. In the free DOX group, the cells were treated with fresh medium containing various concentrations of DOX for 4 h. In the light-treated groups, the cells were treated with fresh medium containing various DOX equivalent concentrations of DOX@HA40-PPys, irradiated with an 810 nm CW laser (1 W cm−2), and incubated for 4 h. Then, the cells were washed two times with medium, and incubated for an additional 44 h. Finally, cell viability was measured using an EnSpire luminometer (PerkinElmer, Waltham, MA, USA). Data are expressed as the mean (SD) of four data samples.
3. Results and discussion
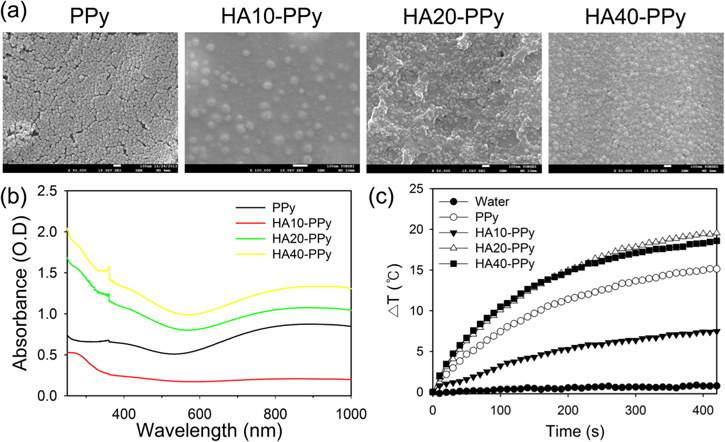
We synthesized PPys and various HA–PPys (HA10-PPy, HA20-PPy, and HA40-PPy) by using a slightly modified version of our previously reported method [21]. SEM images of the prepared nanoparticles (figure 1(a)), showing that the shape and size of all the HA–PPys were similar to those of the PPys, confirmed that the method of doping polypyrrole with HA is appropriate for producing spherical, homogeneous, and well-dispersed nanoparticles. FT-IR spectroscopic and TGA data shown in figure S1 corroborate that the PPy surface was doped with HA. In particular, the relative ratio of the HA bond peak versus PVP bond peak in FT-IR spectroscopy increased with increasing molecular weight of HA, indicating that the PVP on the PPy surface was replaced with the more biocompatible HA during the HA-doping step. Interestingly, the UV/Vis absorbance of both HA20-PPys and HA40-PPys was higher than that of PPys, whereas the UV/Vis absorbance of HA10-PPys was lower than that of the other HA–PPys and PPys (figures 1(b) and S2). Moreover, we found that, upon NIR light irradiation, the photothermal effect displayed by HA20-PPys and HA40-PPys was higher than that displayed by PPys at the same concentration, whereas HA10-PPys did not induce a strong temperature increase (figure 1(c)).
Figure 1. (a) SEM images of PPys and HA–PPys (scale bars: 100 nm). (b) UV/Vis absorption spectra of PPy and HA–PPys at 250 μg ml−1. (c) Temperature increase (ΔT) of PPy and HA–PPys (100 μg ml−1) during light illumination with an 810 nm CW laser at 1 W cm−2.
Download figure:
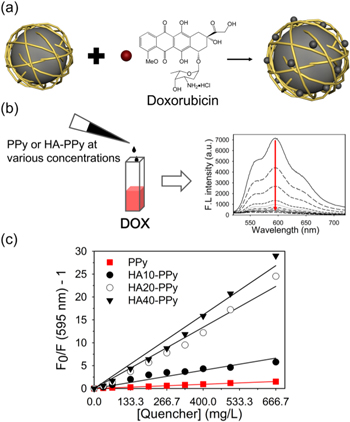
Standard image High-resolution imageNext, we checked the ability of the HA–PPys to quench DOX fluorescence. The surface of HA–PPys is highly negatively charged due to the abundant carboxylic acid groups in HA, and therefore DOX, a positively charged anti-cancer drug, could be incorporated into the nanoparticles via charge–charge interaction (figure 2(a)). Since PPys have high molar extinction coefficients in the visible and NIR regions [15, 16], proximity between DOX and HA–PPy in the drug-loaded nanoparticles is expected to induce strong fluorescence quenching of DOX via fluorescence resonance energy transfer from the excited DOX to polypyrrole, resulting in the fluorescence 'turn-off' of the loaded DOXs. To evaluate fluorescence quenching, DOX in aqueous solution was mixed with PPys or HA–PPys at various concentrations, and then the fluorescence spectra of DOX solutions were analyzed (figure 2(b)). As shown in figure 2(b), fluorescence intensity of DOX diminished sharply with increasing concentration of HA–PPys in the mixed solution, indicating that formation of a charge complex between DOX and the nanoparticles induced fluorescence quenching of DOX. From the Stern–Volmer plots (figure 2(c)), quenching coefficients for PPys, HA10-PPys, HA20-PPys, and HA40-PPys were calculated to be 2.3, 9.9, 33.4, and 40.2 ml mg−1, respectively. It should be noted that the quenching efficiency of HA10-PPys is higher than that of PPys even though UV/Vis absorbance of HA10-PPys is much lower than that of PPys (figures 1 and 2). Taken together, this result supports that the DOX-quenching effect could be obtained not only because of strong UV/Vis absorbance of PPy, but also because of proximity between DOX and PPy after formation of the charge complex between DOX and HA on PPy's surface.
Figure 2. Fluorescence quenching of DOX by PPys and HA–PPys. (a) Illustration showing the formation of a complex between a nanoparticle and DOX, and the subsequent fluorescence quenching of DOX. (b) Fluorescence spectra of DOX solution (10 μg in 100 μl deionized water) mixed with HA–PPys at various concentrations (from top to bottom: 0, 10, 20, 40, 60, 80, 100, 120, 150, and 200 μg in 200 μl). (c) Stern–Volmer plots demonstrating the quenching of DOX fluorescence by HA–PPys. Ksv of PPys (■), HA10-PPys (●), HA20-PPys (○), and HA40-PPys (▼) was calculated to be 2.3, 9.9, 33.4, and 40.2 ml mg−1, respectively.
Download figure:
Standard image High-resolution imageFurthermore, we checked whether HA–PPys could be applied as a nanocarrier and quencher for positively charged NIR fluorochromes. When methylene blue, an FDA-approved NIR dye, was used in the experiment, its fluorescence was extensively quenched, suggesting that HA20-PPys and HA40-PPys have potential utility as quenching agents for positively charged NIR dyes (figure S3).
Based on the UV/Vis spectra, photothermal heating, and fluorescence quenching results, we chose HA40-PPys for further experiments. To determine the loading efficiency of DOX in the nanoparticles, HA40-PPys were mixed with DOX solution at various ratios, and then DOX@HA40-PPys were separated from free DOX. When the purified DOX@HA40-PPys were analyzed (table 1), a 1:10 (w/w) ratio of DOX versus HA40-PPys was shown to be optimal condition for drug loading. A zeta potential of −24.8 mV of the nanoparticles suggests that bound DOX molecules are mainly located deep within the HA layer on the nanoparticle surface (that is, proximal to the PPy core).
Table 1. Effect of DOX versus HA40-PPy ratio on the properties of DOX@HA40-PPys.
| DOX:HA40-PPy ratio (wt/wt %) | Zeta-potential (mV) | DOX loading contents (μM) | DOX loading efficiency (%) |
|---|---|---|---|
| 1:5 | −19.8 | 96.9 ± 4.4a | 7.0a |
| 1:10 | −24.8 | 174.5 ± 7.2a | 25.4a |
| 1:20 | −27.1 | 97.9 ± 5.0a | 28.4a |
| 1:50 | −26.8 | 26.7 ± 12.5b | 19.4b |
| 1:100 | −27.1 | 12.0 ± 5.7b | 17. 4b |
aCalculated from the UV/Vis absorbance of the samples. bBecause of the low UV/Vis absorbance of the samples, these values were calculated from fluorescence intensities of the samples.
Morphology and size of DOX@HA40-PPys (figures 3(a) and S4) were very similar to those of HA40-PPys (figure 1(a)). The absorption spectrum of DOX loaded in HA40-PPys was broader and weaker than that of free DOX (figure 3(b)). The fluorescence intensity of DOX@HA40-PPys was 11-fold weaker than that of free DOX (figure 3(c)). When DOX@HA40-PPys were treated with 5% (w/v) SDS to release DOX from the nanoparticles, the absorption spectrum of the DOX in the nanoparticles became similar to that of free DOX, and the fluorescence intensity of the nanoparticles was greatly recovered (figures S5–6).
Figure 3. (a) SEM image of DOX@HA40-PPys (scale bar: 100 nm). (b) UV/Vis absorption and (c) fluorescence spectra of free DOX and DOX@HA40-PPys (5 μM DOX equivalent). (d) Temperature increase (ΔT) of DOX@HA40-PPys during irradiation with an 810 nm CW laser (light dose rate: 1 W cm−2, concentration: HA40-PPy equivalent).
Download figure:
Standard image High-resolution imageWe then evaluated whether DOX@HA–PPys are stable in the presence of serum (figure S7). Fluorescence quenching of DOX@HA40-PPys was sustained for at least 3 h even when the nanoparticles were dispersed in a PBS solution with high serum content (50% FBS), suggesting that most of the DOX remained in the HA layer and was not released from the nanoparticles. This property is crucial for fluorescence imaging and detection of cancer cells with a high target-to-background ratio in biological conditions. When colloidal stability of DOX@HA40-PPys was tested in various media (figure S8), no apparent aggregation or precipitation was observed for 7 days.
Then, we evaluated whether the photothermal effect of polypyrrole is maintained in DOX@HA40-PPys (figure 3(d)). With 810 nm laser at a power of 1 W cm−2, the temperature of the DOX@HA40-PPy solution increased gradually for 400 s, yielding results that are almost identical to that of HA40-PPy (figure S9), which is high enough to induce targeted cell death [22–24].
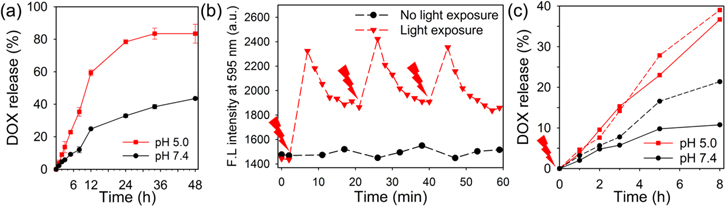
Next, we anticipated that the loaded and quenched status of DOX in the nanoparticles might be sustained since the carboxylic groups of HA are negatively charged at physiological pH (i.e., pH 7.4); however, the preferential accumulation of nanoparticles in tumor tissue via the so-called enhanced permeability and retention effect, followed by endocytosis into cancer cells, might cause the release of DOX from the nanoparticle surface and the subsequent recovery of DOX fluorescence due to the loss of the negative charge of HA at lysosomal pH (pH < 5). Hence, at acidic pH, the charge–charge interaction between DOX and carboxylic groups of HA should become weaker because of the partial neutralization of carboxylic groups at a higher hydrogen ion concentration, thereby promoting the release of DOX from DOX@HA40-PPys. As expected, at pH 7.4, DOX@HA40-PPys released only 43% of DOX after 48 h, but at acidic pH, it took no more than 12 h to release approximately 60% of DOX (figure 4(a)). After 4 h, 83% of DOX was released at acidic pH. These results suggest that the release of DOX is significantly reduced in neutral pH conditions such as in blood, but after endocytosis of the nanoparticles, the release of DOX could be facilitated in lysosomes of cancer cells.
Figure 4. (a) pH-dependent release profile of DOX from DOX@HA40-PPys (n = 3). (b) Fluorescence intensity change of DOX@HA40-PPys upon repeated light irradiation. A red arrow indicates light irradiation for 5 min with 810 nm CW laser (light dose rate: 1 W cm−2). (c) Light-induced DOX release from DOX@HA40-PPys in pH 5.0 and 7.4 (80 μM DOX equivalent). Before dialysis, DOX@HA40-PPy solution was exposed to 810 nm laser at 1 W cm−2 for 5 min (dashed line). DOX@HA40-PPy solutions without light illumination (solid line) are shown for comparison.
Download figure:
Standard image High-resolution imageFurthermore, we monitored changes in the fluorescence intensity of DOX@HA40-PPys upon light irradiation (figure 4(b)) to determine whether we could achieve photo-stimulated drug release at target sites, as has been reported for other systems [25–27]. Surprisingly, without light irradiation, the fluorescence of DOX@HA40-PPys was maintained for 60 min, while with light irradiation for 5 min, the fluorescence of DOX@HA40-PPys was increased by 1.6 fold. Partial dequenching might be due to the release of certain amounts of DOX from the nanoparticles, and this phenomenon, light-induced drug release, could be explained by enhanced drug release from NIR light-absorbed nanomaterials in response to a local thermal stimulus [28]. The slow recovery of quenching of DOX as time elapsed was apparently due to the rebinding of DOX with HA–PPys in a closed test system. In an open system (figure 4(c)), at pH 5.0, the release of DOX was not significantly enhanced by light irradiation. Release percent of DOX at 8 h was slightly increased from 36.7% (without light, solid line) to 39% (with light, dashed line). In contrast, at pH 7.4, a 2-fold increase in DOX release at 8 h was observed in the light-treated DOX@HA40-PPys as compared with that of untreated nanoparticles (10.7% without light versus 21% with light). These data suggest that, in the region of light exposure, DOX release from nanoparticles would be facilitated mainly in the extracellular space of target tumors, while drug release would be curbed in the circulation.
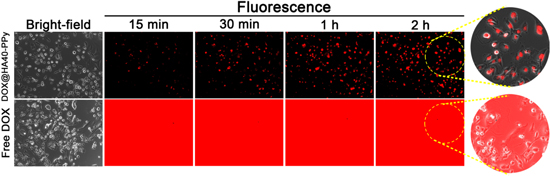
To demonstrate the utility of DOX@HA40-PPys in the real-time fluorescence imaging of TNBC cells, an in vitro live cell imaging study was conducted (figure 5). We expected that the fluorescence of DOX@HA40-PPys would remain quenched in the extracellular region (i.e., at pH 7.4), whereas its fluorescence may be turned on inside lysosomes (i.e., at pH 5.0) of cancer cells because of the preferential release of DOX from nanoparticles as shown in figure 4(a), enabling the detection of cancer cells with a high target-to-background ratio. This is a critical feature for the successful detection of cancer cells because, under physiological conditions, the administered nanoparticles would remain in the circulatory system for a long time [29–32] and could not be manually washed out as in an in vitro experiment, which decreases image contrast. When we obtained fluorescence images in real time without washing the cells, MDA-MB-231 cells treated with DOX@HA40-PPys showed minor extracellular fluorescence during 2 h of incubation, while the intracellular fluorescence intensity gradually increased (particularly in the nuclei, where released DOX intercalates with DNA) with time. This property allowed us to clearly determine the location of cancer cells in fluorescence images. In contrast, strong fluorescence signals were generated in the extracellular region of free DOX-treated MDA-MB-231 cells because the fluorescence of free DOX is always turned on; therefore, it was difficult to determine the location of cancer cells because of high background signals. These results illustrated that DOX@HA40-PPys have potential as a fluorescence-imaging agent for TNBC.
Figure 5. Live cell images of MDA-MB-231 incubated with either free DOX or DOX@HA40-PPys (equivalent of 10 μM of DOX). Fluorescence images were acquired every 15 min in a selected area without washing the cells to confirm the selective fluorescence recovery of DOX@HA40-PPys inside cells. The red signal represents the fluorescence of DOX.
Download figure:
Standard image High-resolution imageNext, prior to determining the cytotoxicity of DOX@HA40-PPys, the biocompatibility of HA40-PPys was evaluated by incubating MDA-MB-231 cells with various concentrations of HA40-PPys for 24 h (figure S10). HA40-PPys showed negligible toxicity even at a concentration of 250 μg ml−1, confirming its excellent biocompatibility.
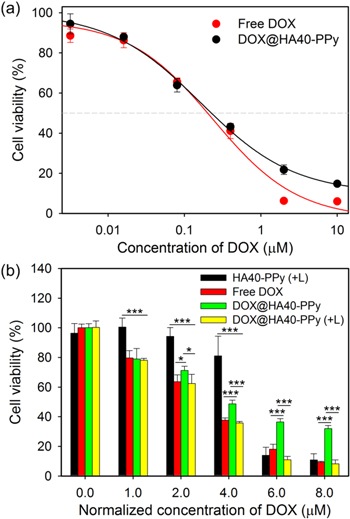
We then evaluated the cytotoxicity of free DOX, HA40-PPys, and DOX@HA40-PPys with or without light illumination in MDA-MB-231 cells (figure 6). At first, we evaluated therapeutic effects of free DOX and DOX@HA40-PPys in the absence of light illumination by treating cells for 24 h with various drug concentrations. Incubation of cells with DOX@HA40-PPys for 24 h was sufficient for intracellular nanoparticle uptake and subsequent drug release inside cells. Cells were treated with free DOX and DOX@HA40-PPys, washed twice with cell-culture medium, and further incubated for 44 h to allow sufficient time for the observation of the DOX anti-proliferative activity (figure 6(a)). The IC50 values of free DOX and DOX@HA40-PPys were very similar suggesting that DOX@HA40-PPys and free DOX are roughly equivalent in terms of their chemotherapeutic effect on TNBC cells. We then hypothesized that chemo/photothermal dual therapy using DOX@HA40-PPys is effective in TNBC treatment, and that photo-stimulated drug release could induce further cell death. To test this hypothesis, cells were incubated with free DOX and DOX@HA40-PPys for 4 h, followed by washing two times with cell culture media. Then, cell viability was measured after incubation for an additional 44 h (figure 6(b)). In the light-treated group, cells were incubated with DOX@HA40-PPys for 4 h, and irradiated with an 810 nm CW laser at 1 W cm−2 for 5 min. As shown in figure 6(b), the cytotoxicity of DOX@HA40-PPys was significantly enhanced upon light irradiation, and the difference in cytotoxicity between light-treated and non-treated groups increased with increasing concentration of nanoparticles, possibly because of the higher photothermal effect and light-induced drug uptake at the higher concentration of HA40-PPys (see figures 3(d) and 7). Geometric means of DOX fluorescence in the cells were increased upon light irradiation, and the increase in mean fluorescence upon light irradiation was 11% at 2 μM DOX equivalent and 21% at 8 μM DOX equivalent. This dose-dependent increase in DOX uptake upon light treatment was likely due to the larger photothermal effect at the higher nanoparticle concentration as shown in figure S9(a). In addition, in figure 6(b), compared to the free DOX-treated cells, the DOX@HA40-PPy-treated cells showed less toxicity, which is similar to results of previous studies using a stimuli-responsive anti-cancer drug nanocarrier system [33, 34], indicating that the sustained pH-dependent release behavior of DOX@HA40-PPys might be beneficial to reduce side effects of DOX such as cardiomyopathy.
Figure 6. (a) Viability of MDA-MB-231 cells treated with free DOX or DOX@HA40-PPys at various DOX-equivalent concentrations for 24 h (n = 4). Cell viability was measured 44 h after washing the cells. IC50 values of DOX and DOX@HA40-PPys were calculated as 0.2 and 0.22 μM, respectively. (b) Viability of MDA-MB-231 cells treated with free DOX, DOX@HA40-PPys, and HA40-PPys (equivalent to HA40-PPy concentrations in DOX@HA40-PPy solution) with and without light illumination. In the light-treated groups, cells were irradiated (+L) with 810 nm CW laser (light dose rate: 1 W cm−2) for 5 min at the initial time point. After 4 h of incubation, cells were washed twice and further incubated for 44 h, following which cell viability was measured (n = 4). *p < 0.05, *** < 0.001.
< 0.001.
Download figure:
Standard image High-resolution imageFigure 7. Flow cytometric analysis of light-stimulated DOX release and subsequent enhanced drug uptake into MDA-MB-231 cells (Ex. 488 nm, Em. 700/54 nm). Cells were non-irradiated (un-filled, line) or irradiated (filled) for 5 min.
Download figure:
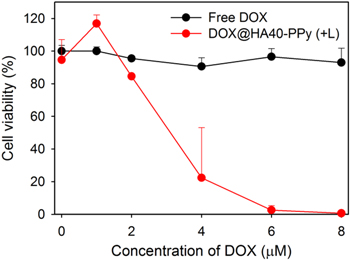
Standard image High-resolution imageAs mentioned above, the long-term exposure of TNBC to DOX leads to a drug-resistant cellular phenotype in tumors. Considering that chemotherapy is currently the only option for TNBC treatment, additional photothermal therapy might be useful for killing drug-resistant TNBC cells within TNBC tumor masses. To demonstrate the photothermal effect of DOX@HA40-PPys on chemoresistant cells, DOX-resistant MDA-MB-231 cells (MDA-MB-231/DOX) were prepared and treated with free DOX or DOX@HA40-PPys. As shown in figure 8, free DOX-treated MB-231/DOX cells showed negligible cytotoxicity, whereas DOX@HA40-PPys with light irradiation induced tremendous cell death at 4 μM DOX equivalent, and complete cell death was achieved at 8 μM DOX equivalent. This significant cell death is mainly due to the photothermal effect of HA40-PPys (figure S11). These results suggest many potential uses for photothermal therapy in overcoming chemoresistance in TNBC.
Figure 8. Cytotoxicity of free DOX and DOX@HA40-PPys in MDA-MB-231/DOX cells. The cells in the DOX@HA40-PPy-treated group were irradiated (+L) with an 810 nm CW laser (light dose rate: 1 W cm−2) for 5 min at the initial time point. After 4 h of incubation with the drugs, cells were washed twice and further incubated for 44 h, following which cell viability was measured (n = 4).
Download figure:
Standard image High-resolution image4. Conclusion
In conclusion, we developed HA–PPys with various molecular weights of HA (HA10-PPy, HA20-PPy, and HA40-PPy) and demonstrated that HA20-PPys and HA40-PPys produced a more robust photothermal effect than non-HA-doped PPys, and had the ability to quench the fluorescence of a positively charged anti-cancer drug (DOX) and fluorochrome (methylene blue). Measurement of the photothermal effect of DOX@HA40-PPys demonstrated its potency as a photothermal and chemotherapeutic agent, and in vitro release experiments demonstrated its pH-dependent and light-induced release properties. The potential utility of these nanoparticles in activatable fluorescence imaging of cancer cells was demonstrated in live cell imaging experiments without washing steps. Furthermore, the viability of cells incubated with a high amount of HA40-PPys (250 μg ml−1) for 24 h showed its excellent biocompatibility, demonstrating its potential for use as a biomaterial. Chemo/photothermal dual therapy using DOX@HA40-PPys was meticulously confirmed by a cytotoxicity assay, with diverse experimental conditions, of TNBC cells. In particular, we illustrated the potential usefulness of the photothermal effect of HA40-PPys for overcoming chemoresistance in TNBC, which is critical given that conventional chemotherapy is currently the only treatment option. Therefore, in this study, we demonstrated the triple functionality of HA40-PPys as a photothermal agent, quencher, and nanocarrier. Furthermore, we demonstrated the utility of DOX@HA40-PPys as a theranostic nanomedicine with applicability in activatable fluorescence imaging and chemo/photothermal therapeutics for the treatment of TNBC.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant (NRF-2014R1A2A1A11050923 and 2015M2A2A6A01044298) funded by the Korea government (MSIP), and by a National Cancer Center grant (1410676), Republic of Korea.