Abstract
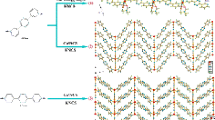
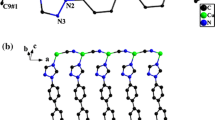
Under hydro(solvo)thermal conditions, four organic bidentate bridging N,N′-donor ligands 1,3-bis(2-methylimidazol-1-yl)propane (L1), 4,4′-di(1H-imidazol-1-yl)-1,1′-biphenyl (L2), 1,2-bis(2-methyl-1H-imidazol-1-ylmethyl)benzene (L3) and 5,6,7,8-tetrahydroquinoxaline (L4) were employed to react with CuBr/CuI, generating four 2-D layered copper(I)–polymer coordination polymer materials [Cu2Br2(L1)] 1, [CuI(L2)] 2, [CuI(L3)] 3 and [CuI(L4)0.5] 4. In 1–4, different Cu–X motifs are found: a cubic Cu4Br4 core in 1; a castellated Cu–I single chain in 2; a rhombic Cu2I2 core in 3; and a staircase-like Cu–I double chain in 4. The 2-D layer networks of 1–3 can all be simplified into a simple 44 topology (planar for 1 and 3; wave-like for 2), while the 2-D layer network of 4 has a 63 topology. The photoluminescence behaviors of 1–4 under a UV lamp suggest that 1 and 2 possess fluorescence thermochromism properties. Under the UV lamp, with the decrease in temperature, (i) 1 exhibits a yellow-to-red emission; (ii) 2 exhibits a yellow-to-green emission; (iii) 3 always emits green light; and (iv) 4 never emits light. These are further confirmed by their emission spectra. From 297 K to 77 K, the emission of 1 exhibits a large red shift from 561 nm to 623 nm; the emission of 2 exhibits a large blue shift from 571 nm to 515 nm; only a minor red shift is observed for the emission of 3; and no peaks appear in the emission spectra of 4. The crystal data of 1 and 2 at different temperatures have been collected for revealing the origination of their fluorescence thermochromism properties. Based on the above investigations, the effect of the rigidity/flexibility of the organic ligand on the fluorescence thermochromism properties of copper(I)–polymer coordination polymer materials is discussed. The quantum yields at 297 K and the photoluminescence lifetimes at 297 K and 77 K for 1–3 were also measured for better understanding their photoluminescence properties.
Similar content being viewed by others
References
(a) R. Peng, M. Li and D. Li, Coord. Chem. Rev., 2010, 254, 1–18; (b) W. H. Fang and G. Y. Yang, J. Solid State Chem., 2014, 212, 249–257; (c) D. Yadav, R. K. Siwatch and S. Soumen, Inorg. Chem., 2014, 53, 600–606; (d) K. M. Henline, C. Wang, R. D. Pike, J. C. Ahern and H. H. Patterson, Cryst. Growth Des., 2014, 14, 1449–1458; (e) Y. Yu, X. Y. Zhang, J. P. Ma, Q. K. Liu, P. Wang and Y. B. Dong, Chem. Commun., 2014, 50, 1444–1446; (f) W. J. Gee and S. R. Batten, Cryst. Growth Des., 2013, 13, 2335–2343; (g) W. H. Fang and G. Y. Yang, CrystEngComm, 2014, 16, 1885–1892; (h) K. Škoch, I. Císařová and P. Štěpnička, Inorg. Chem., 2014, 53, 568–577; (i) V. J. Argyle, M. Roxburgh and L. R. Hanton, CrystEngComm, 2014, 16, 6345–6353; (j) A. Rashid, G. S. Ananthnag, S. Naik, J. T. Mague, D. Panda and M. S. Balakrishna, Dalton Trans., 2014, 43, 11339–11351.
(a) S. Yao, X. D. Sun, B. Bing, R. Krishna, G. H. Li, Q. H. Huo and Y. L. Liu, J. Mater. Chem. A, 2016, 4, 15081–15087; (b) Z. Z. Xue, Z. Zhang, J. Pan, S. D. Han, J. H. Li and G. M. Wang, Dalton Trans., 2017, 46, 13952–13956; (c) Y. Kang, F. Wang, J. Zhang and X. H. Bu, J. Am. Chem. Soc., 2012, 134, 17881–17884.
(a) B. J. Xin, G. Zeng, L. Gao, Y. Li, S. H. Xing, J. Hua, G. H. Li, Z. Shi and S. H. Feng, Dalton Trans., 2013, 42, 7562–7568; (b) A. Tarassoli, V. Nobakht, E. Baladi, L. Carlucci and D. M. Proserpio, CrystEngComm, 2017, 19, 6116–6126; (c) Y. Han, N. F. Chilton, M. Li, C. Huang, H. Xu, H. W. Hou, B. Moubaraki, S. K. Langley, S. R. Batten, Y. T. Fan and K. S. Murray, Chem. –, Eur. J., 2013, 19, 6321–6328.
(a) Z. X. Fu, J. Lin, L. Wang, C. Li, W. B. Yan and T. Wu, Cryst. Growth Des., 2016, 16, 2322–2327; (b) K. Yang, S. H. Li, F. Q. Zhang and X. M. Zhang, Inorg. Chem., 2016, 55, 7323–7325; (c) S. L. Li, J. Wang, F. Q. Zhang and X. M. Zhang, Cryst. Growth Des., 2017, 17, 746–752; (d) K. Kirakci, K. Fejfarová, J. Martinčí, M. Nikl and K. Lang, Inorg. Chem., 2017, 56, 4609–4614; (e) S. S. Zhao, L. Wang, Y. J. Liu, L. Chen and Z. G. Xie, Inorg. Chem., 2017, 56, 13975–13981; (f) S. Q. Bai, D. Kai, K. L. Ke, M. Lin, L. Jiang, Y. Jiang, D. J. Young, X. L. Loh, X. Li and T. S. A. Hor, ChemPlusChem, 2015, 80, 1235–1240; (g) E. Kwon, J. Kim, K. Y. Lee and T. H. Kim, Inorg. Chem., 2017, 56, 943–949; (h) W. Liu, K. Zhu, S. J. Teat, B. J. Deibert, W. B. Yuan and J. Li, J. Mater. Chem. C, 2017, 5, 5962–5969; (i) Q. L. Tang, J. Zhou, F. A. A. Paz, L. Fu, H. Xiao, Q. Zhou and J. Li, Dalton Trans., 2017, 46, 1372–1376; (j) H. Park, E. Kwon, H. Chiang, H. Im, K. Y. Lee, J. Kim and T. H. Kim, Inorg. Chem., 2017, 56, 8287–8294; (k) A. H. Sun, S. D. Han, J. Pan, J. H. Li, G. M. Wang and Z. H. Wang, Cryst. Growth Des., 2017, 17, 3588–3591; (l) J. C. Li, H. X. Li, H. Y. Li, W. J. Gong and J. P. Lang, Cryst. Growth Des., 2016, 16, 1617–1625; (m) S. Q. Bai, L. Jiang, A. L. Tan, S. C. Yeo, D. J. Younga and T. S. A. Hor, Inorg. Chem. Front., 2015, 2, 1011–1018; (n) S. Q. Bai, L. Jiang, D. J. Younga and T. S. A. Hor, Dalton Trans., 2015, 44, 6075–6081; (o) S. Q. Bai, L. Jiang, B. Sun, D. J. Youngac and T. S. A. Hor, CrystEngComm, 2015, 17, 3305–3311; (p) K. A. Vinogradova, V. F. Plyusnin, A. S. Kupryakov, M. I. Rakhmanova, N. V. Pervukhina, D. Y. Naumov, L. A. Sheludyakova, E. B. Nikolaenkova, V. P. Krivopalovd and M. B. Bushuev, Dalton Trans., 2014, 43, 2953–2960; (q) Z. Y. Zhang, Z. P. Deng, X. F. Zhang, L. H. Huo, H. Zhao and S. Gao, CrystEngComm, 2013, 16, 359–368; (r) J. Ni, K. J. Wei, Y. Z. Min, Y. W. Chen, S. Z. Zhan, D. Li and Y. Z. Liu, Dalton Trans., 2012, 41, 5280–5293.
(a) J. Liu, F. Wang, L. Y. Liu and J. Zhang, Inorg. Chem., 2016, 55, 1358–1360; (b) L. X. Hu, M. Y. Gao, T. Wen, Y. Kang and S. M. Chen, Inorg. Chem., 2017, 56, 6507–6511; (c) X. J. Hong, X. Liu, J. B. Zhang, C. L. Lin, X. Wu, Y. J. Ou, J. Yang, H. G. Jin and Y. P. Cai, CrystEngComm, 2014, 16, 7926–7932; (d) X. W. Lei, C. Y. Yue, S. Wang, H. Gao, W. Wang, N. Wing and Y. D. Yin, Dalton Trans., 2017, 46, 4209–4217; (e) T. L. Yu, J. J. Shen, Y. L. Wang and Y. L. Fu, Eur. J. Inorg. Chem., 2015, 1989–1996.
(a) X. L. Wang, M. J. Liu, Y. Q. Wang, H. Y. Fan, J. Wu, C. Huang and H. W. Hou, Inorg. Chem., 2017, 56, 13329–13336; (b) L. Jiang, Z. Wang, S. Q. Bai and T. S. A. Hor, Dalton Trans., 2013, 42, 9437–9443; (c) R. Cargnelutti, F. D. D. Siliva, U. Abram and E. S. Lang, New J. Chem., 2015, 39, 7948–7953; (d) H. X. Zhao, X. X. Li, J. Y. Wang, L. Y. Li and R. H. Wang, ChemPlusChem, 2013, 78, 1491–1502.
(a) S. Perruchas, X. F. Goff, S. Maron, I. Maurin, F. Guillen, A. Garcia, T. Gacoin and J. P. Boilot, J. Am. Chem. Soc., 2010, 132, 10967–10969; (b) D. M. Zink, T. Baumann, J. Friedrichs, M. Nieger and S. Bräse, Inorg. Chem., 2013, 52, 13509–13520; (c) M. S. Deshmukh, A. Yadav, R. Pant and R. Boomishankar, Inorg. Chem., 2015, 54, 1337–1345; (d) S. L. Li and X. M. Zhang, Inorg. Chem., 2014, 53, 8376–8383; (e) F. Farinella, L. Maini, P. P. Mazzeo, V. Fattori, F. Monti and D. Braga, Dalton Trans., 2016, 45, 17939–17947; (f) Y. Song, R. Q. Fan, P. Wang, X. M. Wang, S. Gao, X. Du, X. L. Yang and T. Z. Luan, J. Mater. Chem. C, 2015, 3, 6249–6259; (g) S. L. Li, F. Q. Zhang and X. M. Zhang, Chem. Commun., 2015, 51, 8062–8065; (h) B. C. Tzeng, A. Chao, T. Selvam and T. Y. Chang, CrystEngComm, 2013, 15, 5337–5344; (i) T. Hayashi, A. Kobayashi, H. Ohara, M. Yoshida, T. Matsumoto, H. Chang and M. Kato, Inorg. Chem., 2015, 54, 8905–8913; (j) N. M. Khatri, M. H. Pablico-Lansigan, W. L. Boncher, J. E. Mertzman, A. C. Labatete, L. M. Grande, D. Wunder, M. J. Prushan, W. Zhang, P. S. Halasyamani, J. H. S. K. Monteiro, A. D. Bettencourt-Dias and S. L. Stoll, Inorg. Chem., 2016, 55, 11408–11417; (k) S. Z. Zhan, M. Li, J. Zheng, Q. J. Wang, S. W. Ng and D. Li, Inorg. Chem., 2017, 56, 13446–13455; (l) X. C. Shan, F. L. Jiang, D. Q. Yuan, H. B. Zhang, M. Y. Wu, L. Chen, J. Wei, S. Q. Zhang, J. Pan and M. C. Hong, Chem. Sci., 2013, 4, 1484–1489; (m) J. H. Wang, M. Li, J. Zheng, X. C. Huang and D. Li, Chem. Commun., 2014, 50, 9115–9118; (n) F. S. Wu, H. B. Tong, Z. Y. Li, W. Lei, L. Liu, W. Y. Wong, W. K. Wong and X. J. Zhu, Dalton Trans., 2014, 43, 12463–12466; (o) L. Maini, D. Braga, P. P. Mazzeo, L. Maschio, M. Rérat, I. Manete and B. Ventura, Dalton Trans., 2015, 44, 13003–13006.
(a) K. R. Kyle, C. K. Ryu, J. A. DiBenedetto and P. C. Ford, J. Am. Chem. Soc., 1991, 113, 1954–2965; (b) P. C. Ford, E. Cariati and J. Bourassa, Chem. Rev., 1999, 99, 3625–3647; (c) P. C. Ford and A. Vogler, Acc. Chem. Res., 1993, 26, 220–226; (d) M. Vitale and P. C. Ford, Coord. Chem. Rev., 2001, 219–221, 3–16.
(a) L. P. Xue, X. H. Chang, L. F. Ma and L. Y. Wang, RSC Adv., 2014, 4, 60883–60890; (b) R. Y. Wang, X. Zhang, Q. F. Yang, Q. S. Huo, J. H. Yu, J. N. Xu and J. Q. Xu, J. Solid State Chem., 2017, 251, 176–185.
G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122.
A. J. Black, N. R. Brooks, N. R. Champness, P. A. Cooke, A. M. Deveson, D. Fenske, P. Hubberstey, W. S. Li and M. Schröder, J. Chem. Soc., Dalton Trans., 1999, 2103–2110.
(a) Y. R. Qiao, P. F. Hao and Y. L. Fu, Inorg. Chem., 2015, 54, 8705–8710; (b) D. Braga, L. Maini, P. P. Mazzeo and B. Ventura, Chem. – Eur. J., 2010, 16, 1553–1559; (c) D. Sun, S. Yuan, H. Wang, H. F. Lu, S. Y. Feng and D. F. Sun, Chem. Commun., 2013, 49, 6152–6154; (d) S. Yuan, H. Wang, D. X. Wang, H. F. Lu, S. Y. Feng and D. Sun, CrystEngComm, 2013, 15, 7792–7802; (e) S. Yuan, S. S. Liu and D. Sun, CrystEngComm, 2014, 16, 1927–1933.
X. F. Sun, H. Pan and L. X. Ju, Chin. J. Struct. Chem., 2016, 9, 1406–1412.
F. Y. Yi, J. P. Li, D. Wu and Z. M. Sun, Chem. – Eur. J., 2015, 21, 11475–11482.
X. W. Zhang, P. Q. Xing, X. J. Geng, D. F. Sun, Z. Y. Xiao and L. Wang, J. Solid State Chem., 2015, 229, 49–61.
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic supplementary information (ESI) available. CCDC 1818389 (173 K) for 1, 1818392 for 3 (149 K), 1818394 (173 K) for 2, 1837154 for 4 (293 K), 1837238 (299 K) and 1837240 (297 K). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8pp00474a
Rights and permissions
About this article
Cite this article
Wang, RY., Zhang, X., Yu, JH. et al. Copper(I)–polymers and their photoluminescence thermochromism properties. Photochem Photobiol Sci 18, 477–486 (2019). https://doi.org/10.1039/c8pp00474a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c8pp00474a