Abstract
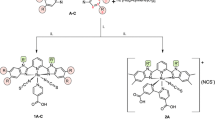
The preparation of a series of new heteroleptic ruthenium complexes containing a 4′-phosphonic acid terpyridine and a terpyridine substituted in 4′osition by a bithiophene or tert-thiophene are described. The UV-Vis absorption and emission spectra along the redox potentials of these complexes are reported. These new complexes were also tested as sensitizers in dry dye-sensitised solar cell using regioregular polyoctylthiophene as a solid hole conductor. It has been shown that the complexes substituted with thiophene chain exhibit poor photovoltaic efficiency most probably due to low electron injection efficiency. This latter property can be rationalised by the fact that the LUMO orbitals in these complexes are localised in the terpyridine substituted by the thiophene chain and not in the terpyridine phosphonic which is bonded to the TiO2 photoanode.
Similar content being viewed by others
References
G. Smestad, C. Bignozzi and R. Argazzi, Testing of dye sensitized TiO2 solar cells I: Experimental photocurrent output and conversion efficiencies, Sol. Energy Mater. Sol. Cell, 1994, 32, 259–272
A. Hagfeldt and M. Grätzel, Light-induced redox reactions in nanocrystalline systems, Chem. Rev., 1995, 95, 49
K. Kalyanasundaram and M. Grätzel, Applications of functionalized transition metal complexes in photonic and optoelectronic devices, Coord. Chem. Rev., 1998, 177, 347–414.
B. O’Regan and M. Grätzel, A low-cost, high-efficiency solar cell based on dye-sensitized colloidal titanium dioxide films, Nature, 1991, 353, 737–740.
U. Bach, D. Lupo, P. Comte, J. E. Moser, F. Weissortel, J. Salbeck, H. Spreitzer and M. Grätzel, Solid-state dye-sensitized mesoporous TiO2 solar cells with high photon-to-electron conversion efficiencies, Nature, 1998, 395, 583–585
K. R. Haridas, J. Ostrauskaite, M. Thelakkat, M. Heim, R. Bilke and D. Haarer, Synthesis of low melting hole conductor systems based on triarylamines and application in dye sensitized solar cells, Synth. Met., 2001, 121, 1573–1574
C. Jäger, R. Bilke, M. Heim, D. Haarer, H. Karickal and M. Thelakkat, Hybrid solar cells with novel hole transporting poly(triphenyldiamine)s, Synth. Met., 2001, 121, 1543–1544
S. Luzzati, M. Basso, M. Catellani, C. J. Brabec, D. Gebeyehu and N. S. Sariciftci, Photo-induced electron transfer from a dithienothiophene-based polymer to TiO2, Thin Solid Films, 2002, 403–404, 52–56
B. O’Regan and D. T. Schwartz, Large Enhancement in Photocurrent Efficiency Caused by UV Illumination of the Dye-Sensitized Heterojunction TiO2/RuLLNCS/CuSCN: Initiation and Potential Mechanisms, Chem. Mater, 1998, 10, 1501–1509
V. P. S. Perera and K. Tennakone, Recombination processes in dye-sensitized solid-state solar cells with CuI as the hole collector, Sol. Energy Mater. Sol. Cells, 2003, 79, 249–255
S. Nagamatsu, S. S. Pandey, W. Takashima, T. Endo, M. Rikukawa and K. Kaneto, Photocarrier transport in processable poly(3-alkylthiophene), Synth. Met., 2001, 121, 1563–1564
K. A. Walters, L. Trouillet, S. Guillerez and K. S. Schanze, Photophysics and Electron Transfer in Poly(3-octylthiophene) Alternating with Ru(II)- and Os(II)-Bipyridine Complexes, Inorg. Chem., 2000, 39, 5496–5509
S. Spiekermann, G. Smestad, J. Kowalik, L. M. Tolbert and M. Grätzel, Poly(4-undecyl-2,2’-bithiophene) as a hole conductor in solid state dye sensitized titanium dioxide solar cells, Synth. Met., 2001, 121, 1603–1604
M. Kinoshita, N. Fujii, T. Tsuzuki and Y. Shirota, Creation of novel light sensitive amorphous molecular materials and their photovoltaic properties, Synth. Met., 2001, 121, 1571–1572.
D. Gebeyehu, C. J. Brabec, F. Padinger, T. Fromherz, S. Spiekermann, N. Vlachopoulos, F. Kienberger, H. Schindler and N. S. Sariciftci, Solid state dye-sensitized TiO2 solar cells with poly(3-octylthiophene) as hole transport layer, Synth. Met., 2001, 121, 1549–1550
D. Gebeyehu, C. J. Brabec and N. S. Sariciftci, Solid-state organic/inorganic hybrid solar cells based on conjugated polymers and dye-sensitized TiO2 electrodes, Thin Solid Films, 2002, 403–404, 271–274.
G. P. Smestad, S. Spiekermann, J. Kowalik, C. D. Grant, A. M. Schwartzberg, J. Zhang, L. M. Tolbert and E. Moons, A technique to compare polythiophene solid-state dye sensitized TiO2 solar cells to liquid junction devices, Sol. Energy Mater. Sol. Cells, 2003, 76, 85–105.
W. Kubo, T. Kitamura, K. Hanabusa, Y. Wada and S. Yanagida, Quasi-solid-state dye-sensitized solar cells using room temperature molten salts and a low molecular weight gelator, Chem. Commun., 2002, 374–375.
W. Kubo, S. Kambe, S. Nakade, T. Kitamura, K. Hanabusa, Y. Wada and S. Yanagida, Photocurrent-Determining Processes in Quasi-Solid-State Dye-Sensitized Solar Cells Using Ionic Gel Electrolytes, J. Phys. Chem. B, 2003, 107, 4374–4381.
Y. Ren, Z. Zhang, S. Fang, M. Yang and S. Cai, Application of PEO based gel network polymer electrolytes in dye-sensitized photoelectrochemical cells, Sol. Energy Mater. Sol. Cells, 2002, 71, 253–259
G. Katsaros, T. Stergiopoulos, I. M. Arabatzis, K. G. Papadokostaki and P. Falaras, A solvent-free composite polymer/inorganic oxide electrolyte for high efficiency solid-state dye-sensitized solar cells, J. Photochem. Photobiol. A: Chem., 2002, 149, 191–198.
U. Bach, Y. Tachibana, J.-E. Moser, S. A. Haque, J. R. Durrant, M. Grätzel and D. R. Klug, Charge Separation in Solid-State Dye-Sensitized Heterojunction Solar Cells, J. Am. Chem. Soc., 1999, 121, 7445–7446
D. Gebeyehu, C. J. Brabec, N. S. Sariciftci, D. Vangeneugden, R. Kebooms, D. Vanderzande, F. Kienberger and H. Schindler, Hybrid solar cells based on dye-sensitized nanoporous TiO2 electrodes and conjugated polymers as hole transport materials, Synth. Met., 2002, 125, 279–287.
G. Kron, T. Egerter, G. Nelles, A. Yasuda, J. H. Werner and U. Rau, Electrical characterization of dye sensitized nanocrystalline TiO2 solar cells with liquid electrolyte and solid-state organic hole conductor, Thin Solid Films, 2002, 403–404, 242–246.
J. Hjelm, E. C. Constable, E. Figgemeier, A. Hagfeldt, R. Handel, C. E. Housecroft, E. Mukhtar and E. Schofield, A rod-like polymer containing Ru(terpy)2 units prepared by electrochemical coupling of pendant thienyl moieties, Chem. Commun., 2002, 284–285
J. Hjelm, R. W. Handel, A. Hagfeldt, E. C. Constable, C. E. Housecroft and R. J. Forster, Conducting Polymers Containing In-Chain Metal Centers: Homogeneous Charge Transport through a Quaterthienyl-Bridged Os(tpy)2 Polymer, J. Phys. Chem. B, 2003, 107, 10431–10439.
K. Murakoshi, R. Kogure, Y. Wada and S. Yanagida, Fabrication of solid-state dye-sensitized TiO2 solar cells combined with polypyrrole, Sol. Energy Mater. Sol. Cells, 1998, 55, 113–125.
E. C. Constable, R. Handel, C. E. Housecroft, M. Neuburger, E. R. Schofield and M. Zehnder, Efficient syntheses of 4’-(2-thienyl)- and 4’-(3-thienyl)-2,2’:6’,2″-terpyridine: preparation and characterization of Fe(II), Ru(II), Os(II) and Co(II) complexes, Polyhedron, 2004, 23, 135–143.
P.-L. Vidal, B. Divisia-Blohorn, G. Bidan, J.-L. Hazemann, J.-M. Kern and J.-P. Sauvage, p-Conjugated ligand polymers entwined around copper centers, Chem.-Eur. J., 2000, 6, 1663–1673.
K. Tamao, S. Kodama, I. Nakajima and M. Kumada, Nickel-phosphine complex catalyzed Grignard coupling-II, Tetrahedron, 1982, 38, 3347–3354.
P. Bäuerle, F. Würthner, G. Go¨tz and F. Effenberger, Selective synthesis of a-substituted oligothiophenes, Synthesis, 1993, 1099–1103
M. A. Keegstra and L. Brandsma, Convenient high-yield procedures for 2-bromothiophene and 2,5-dibromothiophene, Synthesis, 1988, 890–891.
A. F. Littke and G. C. Fu, The first general method for Stille cross-couplings of aryl chlorides, Angew. Chem., Int. Ed., 1999, 38, 2411–2413.
O. Henze, U. Lehmann and A. D. Schlueter, Synthesis of 5,5’-disubstituted 2,2’-bipyridines for modular chemistry, Synthesis, 1999, 683–687
U. Lehmann, O. Henze and A. D. Schluter, 5,5/“-Disubstituted 2,2’:6’,2/”-terpyridines through and for metal-mediated cross-coupling chemistry, Chem. Eur. J., 1999, 5, 854–859
M. Heller and U. S. Schubert, Functionalized2,2’-Bipyridinesand 2,2’:6’,2″-Terpyridines via Stille-Type Cross-Coupling Procedures, J. Org. Chem., 2002, 67, 8269–8272
G. R. Newkome, A. K. Patri, E. Holder and U. S. Schubert, Synthesis of 2,2’-bipyridines: Versatile building blocks for sexy architectures and functional nanomaterials, Eur. J. Org. Chem., 2004, 235–254
U. S. Schubert, C. Eschbaumer and M. Heller, Stille-Type Cross-Coupling-An Efficient Way to Various Symmetrically and Unsymmetrically Substituted Methyl-Bipyridines: Toward New ATRP Catalysts, Org. Lett.,2000, 2, 3373–3376.
M. Beley, J. P. Collin and J. P. Sauvage, Highly coupled mixed-valence dinuclear ruthenium and osmium complexes with a bis-cyclometalating terpyridine analog as bridging ligand, Inorg. Chem., 1993, 32, 4539–4543
F. Barigelletti, L. Flamigni, V. Balzani, J.-P. Collin, J.-P. Sauvage and A. Sour, Luminescence properties of rigid, rod-like, dichromophoric species Dinuclear Ru-Os terpyridine-type complexes with 2.4 nm metal-to-metal distance, New J. Chem., 1995, 19, 793–798.
S. M. Zakeeruddin, M. K. Nazeeruddin, P. Pechy, F. P. Rotzinger, R. Humphry-Baker, K. Kalyanasundaram, M. Grätzel, V. Shklover and T. Haibach, Molecular Engineering of Photosensitizers for Nanocrystalline Solar Cells: Synthesis and Characterization of Ru Dyes Based on Phosphonated Terpyridines, Inorg. Chem., 1997, 36, 5937–5946.
I. Gillaizeau-Gauthier, F. Odobel, M. Alebbi, R. Argazzi, E. Costa, C. A. Bignozzi, P. Qu and G. J. Meyer, Phosphonate-Based Bipyridine Dyes for Stable Photovoltaic Devices, Inorg. Chem., 2001, 40, 6073–6079
H. Zabri, I. Gillaizeau-Gauthier, C. A. Bignozzi, S. Caramori, M.-F. Charlot, J.-C. Boquera and F. Odobel, Synthesis and Comprehensive Characterizations of New cis-RuL2X2 (X = Cl, CN and NCS) Sensitizers for Nanocrystalline TiO2 solar cell using bis-phosphonated bipyridine ligands (L), Inorg. Chem., 2003, 42, 6655–6666.
C. E. McKenna, M. T. Higa, N. H. Cheung and M. C. McKenna, The facile dealkylation of phosphonic acid dialkyl esters by bro-motrimethylsilane, Tetrahedron Lett., 1977, 155–158.
K. Kalyanasundaram in Photochemistry of polypyridine and porphyrin complexes, ed. K. Kalyanasundaram, Academic Press, London, 1992, p. 106–112.
S. Encinas, L. Flamigni, F. Barigelletti, E. C. Constable, C. E. Housecroft, E. R. Schofield, E. Figgemeier, D. Fenske, M. Neuburger, J. G. Vos and M. Zehnder, Electronic energy transfer and collection in luminescent molecular rods containing ruthenium(II) and osmium(II) 2,2:6,2-terpyridine complexes linked by thiophene-2,5-diyl spacers, Chem.-Eur. J., 2002, 8, 137–150.
T. M. Pappenfus and K. R. Mann, Synthesis, spectroscopy and electrochemical studies of binuclear tris-bipyridine ruthenium(II) complexes with oligothiophene bridges, Inorg. Chem., 2001, 40, 6301–6307.
A. Harriman, A. Mayeux, A. De Nicola and R. Ziessel, Synthesis and photophysical properties of ruthenium(II) bis(2,2’:6’,2″-terpyridine) complexes constructed from a diethynylated-thiophene residue, Phys. Chem. Chem. Phys., 2002, 4, 2229–2235.
Lmax of the MLCT transition in complex 10 was at 486 nm and passed to 482 nm for complex 13 after hydrolysis..
E. Figgemeier, V. Aranyos, E. C. Constable, R. W. Handel, C. E. Housecroft, C. Risinger, A. Hagfeldt and E. Mukhtar, Modification of electron transfer properties in photoelectrochemical solar cells by substituting Ru(terpy)22+ dyes with thiophene, Inorg. Chem. Commun., 2003, 7, 117–121.
J. Hjelm, R. W. Handel, A. Hagfeldt, E. C. Constable, C. E. Housecroft and R. J. Forster, Electropolymerisation dynamics of a highly conducting metallopolymer: poly-[Os(4’-(5-(2,2’-bithienyl))-2,2’:6’,2″-terpyridine)2]2+, Electrochem. Commun., 2004, 6, 193–200.
J. Roncali, Conjugated Poly(thiophenes): Synthesis, Functionaliza-tion and applications, Chem. Rev., 1992, 92, 711–738
J. Roncali, Synthetic principles for bandgap control in linear p-conjugated systems, Chem. Rev., 1997, 97, 173–205.
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic supplementary information (ESI) available: Full experimental details with the 1H NMR,13C NMR and mass spectra characterisations of all new materials. See http://www.rsc.org/suppdata/pp/b4/b414031a/
Rights and permissions
About this article
Cite this article
Houarner, C., Blart, E., Buvat, P. et al. Ruthenium bis-terpyridine complexes connected to an oligothiophene unit for dry dye-sensitised solar cells. Photochem Photobiol Sci 4, 200–204 (2005). https://doi.org/10.1039/b414031a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/b414031a