Abstract
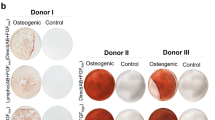
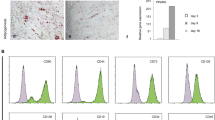
Type I interferon (IFN) is shown to control the reversible quiescence of a primitive human bone marrow mesenchymal stem cell (MSC) subpopulation. A 24 h pre-treatment of Stro1+/GlycoA- or CD45-/GlycoA- subpopulations with a monoclonal antibody (mAb) against the IFNAR1 chain of the human type I IFN receptor (64G12), or with a polyclonal anti-IFNα antibody, resulted in a marked increase in the number of very large colonies (CFU-F >3000 cells) obtained in the presence of low, but necessary, concentrations of bFGF. Over a 2-month culture period, this short activation promoted a faster and greater amplification of mesenchymal progenitors for adipocytes and osteoblasts. Activation correlated with inhibition of STAT1 and STAT2 phosphorylation and of STAT1 nuclear translocation. A non-neutralizing anti-IFNAR1 mAb was ineffective. We demonstrate that control and activated MSCs express ST3GAL3, a sialyltransferase necessary to produce the embryonic antigens SSEA-3 and -4. Interestingly, activated MSC progeny expressed SSEA-3 and -4 at a higher level than control cultures, but this was not correlated with a significant expression of other embryonic markers. As MSCs represent an essential tool in tissue regeneration, the use of 64G12, which rapidly recruits a higher number of primitive cells, might increase amplification safety for cell therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Friedenstein AJ, Chailakhjan RK, Lalykina KS . The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet 1970; 3: 393–403.
Dennis JE, Charbord P . Origin and differentiation of human and murine stroma. Stem Cells 2002; 20: 205–214.
Deschaseaux F, Charbord P . Human marrow stromal precursors are alpha 1 integrin subunit-positive. J Cell Physiol 2000; 184: 319–325.
Majumdar MK, Banks V, Peluso DP, Morris EA . Isolation, characterization, and chondrogenic potential of human bone marrow-derived multipotential stromal cells. J Cell Physiol 2000; 185: 98–106.
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284: 143–147.
Simmons PJ, Torok-Storb B . Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood 1991; 78: 55–62.
Bianco P, Riminucci M, Gronthos S, Robey PG . Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 2001; 19: 180–192.
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 2001; 7: 211–228.
In ‘t Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 2004; 22: 1338–1345.
De Bari C, Dell'Accio F, Tylzanowski P, Luyten FP . Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum 2001; 44: 1928–1942.
Erices A, Conget P, Minguell JJ . Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol 2000; 109: 235–242.
Prockop DJ, Gregory CA, Spees JL . One strategy for cell and gene therapy: harnessing the power of adult stem cells to repair tissues. Proc Natl Acad Sci USA 2003; 100 (Suppl 1): 11917–11923.
Gregory CA, Prockop DJ, Spees JL . Non-hematopoietic bone marrow stem cells: molecular control of expansion and differentiation. Exp Cell Res 2005; 306: 330–335.
Angoulvant D, Clerc A, Benchalal S, Galambrun C, Farre A, Bertrand Y et al. Human mesenchymal stem cells suppress induction of cytotoxic response to alloantigens. Biorheology 2004; 41: 469–476.
Benizri E, Gugenheim J, Lasfar A, Eid P, Blanchard B, Lallemand C et al. Prolonged allograft survival in cynomolgus monkeys treated with a monoclonal antibody to the human type I interferon receptor and low doses of cyclosporine. J Interferon Cytokine Res 1998; 18: 273–284.
Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci USA 2002; 99: 8932–8937.
Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med 1999; 5: 309–313.
Koc ON, Day J, Nieder M, Gerson SL, Lazarus HM, Krivit W . Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH). Bone Marrow Transplant 2002; 30: 215–222.
Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol 2000; 18: 307–316.
Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004; 363: 1439–1441.
Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002; 418: 41–49.
Prockop DJ . Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 1997; 276: 71–74.
Reyes M, Lund T, Lenvik T, Aguiar D, Koodie L, Verfaillie CM . Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood 2001; 98: 2615–2625.
Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T et al. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest 2002; 109: 1291–1302.
Hatzfeld J, Li ML, Brown EL, Sookdeo H, Levesque JP, O'Toole T et al. Release of early human hematopoietic progenitors from quiescence by antisense transforming growth factor beta 1 or Rb oligonucleotides. J Exp Med 1991; 174: 925–929.
Larsson J, Karlsson S . The role of Smad signaling in hematopoiesis. Oncogene 2005; 24: 5676–5692.
Ruscetti FW, Akel S, Bartelmez SH . Autocrine transforming growth factor-beta regulation of hematopoiesis: many outcomes that depend on the context. Oncogene 2005; 24: 5751–5763.
Scandura JM, Boccuni P, Massague J, Nimer SD . Transforming growth factor beta-induced cell cycle arrest of human hematopoietic cells requires p57KIP2 up-regulation. Proc Natl Acad Sci USA 2004; 101: 15231–15236.
Fortunel NO, Hatzfeld JA, Rosemary PA, Ferraris C, Monier MN, Haydont V et al. Long-term expansion of human functional epidermal precursor cells: promotion of extensive amplification by low TGF-beta1 concentrations. J Cell Sci 2003; 116: 4043–4052.
Cardoso AA, Li ML, Batard P, Hatzfeld A, Brown EL, Levesque JP et al. Release from quiescence of CD34+ CD38- human umbilical cord blood cells reveals their potentiality to engraft adults. Proc Natl Acad Sci USA 1993; 90: 8707–8711.
Sansilvestri P, Cardoso AA, Batard P, Panterne B, Hatzfeld A, Lim B et al. Early CD34high cells can be separated into KIThigh cells in which transforming growth factor-beta (TGF-beta) downmodulates c-kit and KITlow cells in which anti-TGF-beta upmodulates c-kit. Blood 1995; 86: 1729–1735.
Ducos K, Panterne B, Fortunel N, Hatzfeld A, Monier MN, Hatzfeld J . p21(cip1) mRNA is controlled by endogenous transforming growth factor-beta1 in quiescent human hematopoietic stem/progenitor cells. J Cell Physiol 2000; 184: 80–85.
Fortunel NO, Hatzfeld A, Hatzfeld JA . Transforming growth factor-beta: pleiotropic role in the regulation of hematopoiesis. Blood 2000; 96: 2022–2036.
Fortunel N, Batard P, Hatzfeld A, Monier MN, Panterne B, Lebkowski J et al. High proliferative potential-quiescent cells: a working model to study primitive quiescent hematopoietic cells. J Cell Sci 1998; 111: 1867–1875.
Fortunel N, Hatzfeld J, Kisselev S, Monier MN, Ducos K, Cardoso A et al. Release from quiescence of primitive human hematopoietic stem/progenitor cells by blocking their cell-surface TGF-beta type II receptor in a short-term in vitro assay. Stem Cells 2000; 18: 102–111.
Gronthos S, Simmons PJ . The growth factor requirements of STRO-1-positive human bone marrow stromal precursors under serum-deprived conditions in vitro. Blood 1995; 85: 929–940.
Lee BS, Stewart EA, Sahakian M, Nowak RA . Interferon-alpha is a potent inhibitor of basic fibroblast growth factor-stimulated cell proliferation in human uterine cells. Am J Reprod Immunol 1998; 40: 19–25.
Oreffo RO, Romberg S, Virdi AS, Joyner CJ, Berven S, Triffitt JT . Effects of interferon alpha on human osteoprogenitor cell growth and differentiation in vitro. J Cell Biochem 1999; 74: 372–385.
Benoit P, Maguire D, Plavec I, Kocher H, Tovey M, Meyer F . A monoclonal antibody to recombinant human IFN-alpha receptor inhibits biologic activity of several species of human IFN-alpha, IFN-beta, and IFN-omega. Detection of heterogeneity of the cellular type I IFN receptor. J Immunol 1993; 150: 707–716.
Eid P, Tovey MG . Characterization of a domain of a human type I interferon receptor protein involved in ligand binding. J Interferon Cytokine Res 1995; 15: 205–211.
eaman DW, Chawla-Sarkar M, Jacobs B, Vyas K, Sun Y, Ozdemir A et al. Novel growth and death related interferon-stimulated genes (ISGs) in melanoma: greater potency of IFN-beta compared with IFN-alpha2. J Interferon Cytokine Res 2003; 23: 745–756.
Darnell Jr JE, Kerr IM, Stark GR . Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 1994; 264: 1415–1421.
Yan H, Krishnan K, Greenlund AC, Gupta S, Lim JT, Schreiber RD et al. Phosphorylated interferon-alpha receptor 1 subunit (IFNaR1) acts as a docking site for the latent form of the 113 kDa STAT2 protein. EMBO J 1996; 15: 1064–1074.
Pochampally RR, Smith JR, Ylostalo J, Prockop DJ . Serum deprivation of human marrow stromal cells (hMSCs) selects for a subpopulation of early progenitor cells with enhanced expression of OCT-4 and other embryonic genes. Blood 2004; 103: 1647–1652.
Fortunel N, Hatzfeld J, Aoustin L, Batard P, Ducos K, Monier MN et al. Specific dose-response effects of TGF-beta1 on developmentally distinct hematopoietic stem/progenitor cells from human umbilical cord blood. Hematol J 2000; 1: 126–135.
Tovey MG, Streuli M, Gresser I, Gugenheim J, Blanchard B, Guymarho J et al. Interferon messenger RNA is produced constitutively in the organs of normal individuals. Proc Natl Acad Sci USA 1987; 84: 5038–5042.
James D, Levine AJ, Besser D, Hemmati-Brivanlou A . TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development 2005; 132: 1273–1282.
Besser D . Expression of nodal, lefty-a, and lefty-B in undifferentiated human embryonic stem cells requires activation of Smad2/3. J Biol Chem 2004; 279: 45076–45084.
Pera MF, Andrade J, Houssami S, Reubinoff B, Trounson A, Stanley EG et al. Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. J Cell Sci 2004; 117: 1269–1280.
Rho JY, Yu K, Han JS, Chae JI, Koo DB, Yoon HS et al. Transcriptional profiling of the developmentally important signalling pathways in human embryonic stem cells. Hum Reprod 2006; 21: 405–412.
Schuldiner M, Yanuka O, Itskovitz-Eldor J, Melton DA, Benvenisty N . Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc Natl Acad Sci USA 2000; 97: 11307–11312.
Vallier L, Alexander M, Pedersen RA . Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci 2005; 118: 4495–4509.
Xu RH, Peck RM, Li DS, Feng X, Ludwig T, Thomson JA . Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods 2005; 2: 185–190.
Giron-Michel J, Weill D, Bailly G, Legras S, Nardeux PC, Azzarone B et al. Direct signal transduction via functional interferon-alphabeta receptors in CD34+ hematopoietic stem cells. Leukemia 2002; 16: 1135–1142.
Pledger WJ, Stiles CD, Antoniades HN, Scher CD . Induction of DNA synthesis in BALB/c 3T3 cells by serum components: reevaluation of the commitment process. Proc Natl Acad Sci USA 1977; 74: 4481–4485.
Stiles CD, Capone GT, Scher CD, Antoniades HN, Van Wyk JJ, Pledger WJ . Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci USA 1979; 76: 1279–1283.
Gang EJ, Bosnakovski D, Figueiredo CA, Visser JW, Perlingeiro RC . SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood 2006 [E-pub ahead of print].
Brimble SN, Sherrer ES, Uhl EW, Wang E, Kelly S, Merrill Jr AH et al. The Cell Surface Glycosphingolipids SSEA-3 and SSEA-4 are not Essential for Human ES Cell Pluripotency. Stem Cells 2007; 1: 54–62.
Rubio D, Garcia-Castro J, Martin MC, de la Fuente R, Cigudosa JC, Lloyd AC et al. Spontaneous human adult stem cell transformation. Cancer Res 2005; 65: 3035–3039.
Acknowledgements
This work was supported by the European Integrated Project (PCRD6) ‘GENOSTEM’: ‘Adult mesenchymal stem cells engineering for connective tissue disorders. From the bench to the bed side’. Contract no: 503161. RB, MLL and IP were appointed by the Genostem Project. We are indebted to Dr Mary Osborne-Pellegrin for editing the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hatzfeld, A., Eid, P., Peiffer, I. et al. A sub-population of high proliferative potential-quiescent human mesenchymal stem cells is under the reversible control of interferon α/β. Leukemia 21, 714–724 (2007). https://doi.org/10.1038/sj.leu.2404589
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2404589
Keywords
This article is cited by
-
The uncertain role of unmodified mesenchymal stem cells in tumor progression: what master switch?
Stem Cell Research & Therapy (2013)
-
Distinct Stem Cells Subpopulations Isolated from Human Adipose Tissue Exhibit Different Chondrogenic and Osteogenic Differentiation Potential
Stem Cell Reviews and Reports (2011)
-
Human Bone Marrow Mesenchymal Stem Cells: A Systematic Reappraisal Via the Genostem Experience
Stem Cell Reviews and Reports (2011)
-
Transcriptional profiling of putative human epithelial stem cells
BMC Genomics (2008)