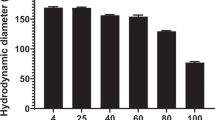
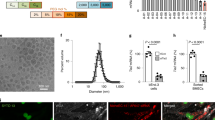
Abstract
We investigated the in vitro and in vivo properties of DNA/transferrin–polyethylenimine (800 kDa) complexes before and after covalent coupling of poly(ethylene glycol) (PEG). Upon incubation with plasma, the positively charged non-PEGylated DNA complexes form aggregates. Plasma proteins such as IgM, fibrinogen, fibronectin and complement C3 were found to bind to non-PEGylated DNA complexes. At DNA concentrations relevant for in vivo gene delivery a strong aggregation of erythrocytes was also observed. PEGylation of the complexes strongly reduces plasma protein binding and erythrocyte aggregation. Furthermore, PEGylated complex size was stabilized and had a reduced surface charge. Prolonged circulation in the blood of the PEGylated complexes was also observed when injected intravenously. In tumor bearing mice, application of non-PEGylated complexes through the tail vein resulted in reporter gene expression in tail and lung, but severe toxicity was observed in some mice. In contrast, PEGylated complexes mediated reporter gene transfer to the tumor without significant toxicity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Chonn A, Semple SC, Cullis PR . Association of blood proteins with large unilamellar liposomes in vivo. Relation to circulation lifetimes J Biol Chem 1992 267: 18759–18765
Senior J et al. Influence of surface hydrophilicity of liposomes on their interaction with plasma protein and clearance from the circulation: studies with poly(ethylene glycol)-coated vesicles Biochim Biophys Acta 1991 1062: 77–82
Mori A, Klibanov AL, Torchilin VP, Huang L . Influence of the steric barrier activity of amphipathic poly(ethyleneglycol) and ganglioside GM1 on the circulation time of liposomes and on the target binding of immunoliposomes in vivo FEBS Lett 1991 284: 263–266
Torchilin VP et al. Amphiphilic vinyl polymers effectively prolong liposome circulation time in vivo Biochim Biophys Acta 1994 1195: 181–184
Woodle MC, Engbers CM, Zalipsky S . New amphipatic polymer-lipid conjugates forming long-circulating reticuloendothelial system-evading liposomes Bioconjug Chem 1994 5: 493–496
Boussif O et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine Proc Natl Acad Sci USA 1995 92: 7297–7301
Abdallah B et al. A powerful nonviral vector for in vivo gene transfer into the adult mammalian brain: polyethylenimine Hum Gene Ther 1996 7: 1947–1954
Ferrari S et al. ExGen 500 is an efficient vector for gene delivery to lung epithelial cells in vitro and in vivo Gene Therapy 1997 4: 1100–1106
Kircheis R et al. Coupling of cell-binding ligands to polyethylenimine for targeted gene transfer Gene Therapy 1997 4: 409–418
Zanta MA, Boussif O, Adib A, Behr JP . In vitro gene delivery to hepatocytes with galactosylated polyethylenimine Bioconjug Chem 1997 8: 839–844
Dunlap DD, Maggi A, Soria MR, Monaco L . Nanoscopic structure of DNA condensed for gene delivery Nucleic Acids Res 1997 25: 3095–3101
Goula D et al. Size, diffusibility and transfection performance of linear PEI/DNA complexes in the mouse central nervous system Gene Therapy 1998 5: 712–717
Ogris M et al. The size of DNA/transferrin–PEI complexes is an important factor for gene expression in cultured cells Gene Therapy 1998 5: 1425–1433
Wolfert MA et al. Characterization of vectors for gene therapy formed by self-assembly of DNA with synthetic block co-polymers Hum Gene Ther 1996 7: 2123–2133
Cotten M et al. Adenovirus polylysine DNA conjugates In: Dracopoli NC et al (eds) . Current Protocols in Human Genetics John Wiley and Sons: New York 1996 pp 12.3.112–.3.33
Needham D, McIntosh TJ, Lasic DD . Repulsive interactions and mechanical stability of polymer-grafted lipid membranes Biochim Biophys Acta 1992 1108: 40–48
Plank C, Mechtler K, Szoka FJ, Wagner E . Activation of the complement system by synthetic DNA complexes: a potential barrier for intravenous gene delivery Hum Gene Ther 1996 7: 1437–1446
Wagner E, Curiel D, Cotten M . Delivery of drugs, proteins and genes into cells using transferrin as a ligand for receptor-mediated endocytosis Adv Drug Del Rev 1994 14: 113–136
Allen TM, Hansen C . Pharmacokinetics of stealth versus conventional liposomes: effect of dose Biochim Biophys Acta 1991 1068: 133–141
Papahadjopoulos D . Fate of liposomes in vivo: a brief introductory review J Liposome Res 1996 6: 3–17
Absolom DR . Opsonins and dysopsonins: an overview Meth Enzymol 1986 132: 281–318
Park YS, Huang L . Effect of chemically modified GM1 and neoglycolipid analogs of GM1 on liposome circulation time: evidence supporting the dysopsonin hypothesis Biochim Biophys Acta 1993 1166: 105–114
Semple SC, Chonn A, Cullis PR . Influence of cholesterol on the association of plasma proteins with liposomes Biochemistry 1996 35: 2521–2525
Semple SC, Chonn A . liposome blood protein interactions in relation to liposome clearence J Liposome Res 1996 6: 32–60
Moghimi SM et al. Coating particles with a block co-polymer (poloxamine-908) suppresses opsonization but permits the activity of dysopsonins in the serum Biochim Biophys Acta 1993 1179: 157–165
Litzinger DC, Huang L . Amphipathic poly(ethylene glycol) 5000-stabilized dioleoylphosphatidylethanolamine liposomes accumulate in spleen Biochim Biophys Acta 1992 1127: 249–254
Torchilin VP et al. Targeted accumulation of polyethylene glycol-coated immunoliposomes in infarcted rabbit myocardium FASEB J 1992 6: 2716–2719
Blume G et al. Specific targeting with poly(ethylene glycol)-modified liposomes: coupling of homing devices to the ends of the polymeric chains combines effective target binding with long circulation times Biochim Biophys Acta 1993 1149: 180–184
Maruyama K et al. Immunoliposomes bearing polyethyleneglycol-coupled Fab′ fragment show prolonged circulation time and high extravasation into targeted solid tumors in vivo FEBS Lett 1997 413: 177–180
Gerlowski LE, Jain RK . Microvascular permeability of normal and neoplastic tissues Microvasc Res 1986 31: 288–305
Unezaki S et al. Direct measurement of the extravasation of polyethyleneglycol-coated liposomes into solid tumor tissue by in vivo fluorescence microscopy Int J Pharmaceutics 1996 144: 11–17
Sadzuka Y, Hirotsu S, Hirota S . Effect of liposomalization on the antitumor activity, side-effects and tissue distribution of CPT-11 Cancer Lett 1998 127: 99–106
Schwartz B et al. Lipospermine based gene transfer into the newborn mouse brain is optimized by a low lipospermine/DNA charge ratio Hum Gene Ther 1995 6: 1515–1524
Li S, Rizzo MA, Bhattacharya S, Huang L . Characterization of cationic lipid-protamine–DNA (LPD) complexes for intravenous gene delivery Gene Therapy 1998 5: 930–937
Tang MX, Szoka FC . The influence of polymer structure on the interactions of cationic polymers with DNA and morphology of the resulting complexes Gene Therapy 1997 4: 823–832
McLean JW et al. Organ-specific endothelial cell uptake of cationic liposome–DNA complexes in mice Am J Physiology Heart Circ Physiol 1997 42: H387–H404
Liu Y et al. Factors influencing the efficiency of cationic liposome-mediated intravenous gene delivery Nat Biotechnol 1997 15: 167–173
Li S, Huang L . In vivo gene transfer via intravenous administration of cationic lipid protamine DNA (LPD) complexes Gene Therapy 1997 4: 891–900
Templeton NS et al. Improved DNA: liposome complexes for increased systemic delivery and gene expression Nat Biotechnol 1997 15: 647–652
Litzinger DC et al. Fate of cationic liposome and their complex with oligonucleotide in vivo Biochim Biophys Acta 1996 1281: 139–149
Liu F, Qi H, Huang L, Liu D . Factors controlling the efficiency of cationic lipid-mediated transfection in vivo via intravenous administration Gene Therapy 1997 4: 517–523
Goula D et al. Polyethylenimine-based intravenous delivery of transgenes to mouse lung Gene Therapy 1998 5: 1291–1295
Plank C et al. The influence of endosome-disruptive peptides on gene transfer using synthetic virus-like gene transfer systems J Biol Chem 1994 269: 12918–12924
Zauner W, Ogris M, Wagner E . Polylysine-based transfection systems utilizing receptor-mediated delivery Adv Drug Delivery Rev 1998 30: 97–113
Sebestyen MG et al. DNA vector chemistry: the covalent attachment of signal peptides to plasmid DNA Nat Biotechnol 1998 16: 80–85
Plank C et al. Gene transfer into hepatocytes using asialoglycoprotein receptor mediated endocytosis of DNA complexed with an artificial tetra-antennary galactose ligand Bioconjug Chem 1992 3: 533–539
Bloom H, Beier H, Gross HS . Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels Electrophoresis 1987 8: 93–99
Miller J, Schätzel B, Vincent J . The determination of very small electrophoretic mobilities in polar and nonpolar colloidal dispersions using phase analasys light scattering J Coll Intersci 1991 143: 532–555
Kircheis R et al. Polycation-based DNA complexes for tumor-targeted gene delivery in vivo J Gene Med 1999 (in press)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ogris, M., Brunner, S., Schüller, S. et al. PEGylated DNA/transferrin–PEI complexes: reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery. Gene Ther 6, 595–605 (1999). https://doi.org/10.1038/sj.gt.3300900
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3300900
Keywords
This article is cited by
-
BBB-on-a-chip with integrated micro-TEER for permeability evaluation of multi-functionalized gold nanorods against Alzheimer’s disease
Journal of Nanobiotechnology (2023)
-
Directing the Way—Receptor and Chemical Targeting Strategies for Nucleic Acid Delivery
Pharmaceutical Research (2023)
-
ApoE—functionalization of nanoparticles for targeted brain delivery—a feasible method for polyplexes?
Drug Delivery and Translational Research (2023)
-
pH-sensitive packaging of cationic particles by an anionic block copolymer shell
Journal of Nanobiotechnology (2022)
-
Targeting the Inside of Cells with Biologicals: Chemicals as a Delivery Strategy
BioDrugs (2021)